Ubiquitin-specific proteases (USPs) represent the most widespread and represented deubiquitinylating enzymes across evolution. USPs tend to release ubiquitin from a conjugated protein. They display similar catalytic domains but divergent N-terminal and occasionally C-terminal extensions, which are thought to function in substrate recognition, subcellular localization, and protein-protein interactions.
USP2 is a ubiquitin-specific protease thought to function at the pre-proteasomal level by preventing the degradation of fatty acid synthase (FAS). FAS overexpression occurs in the majority of epithelial tumors, including prostate cancer, and protects cancer cells from apoptosis. Because USP2 is overexpressed in prostate cancer and its inhibition results in apoptosis of transformed prostatic cells, this isopeptidase is a potential target for induction of apoptosis in prostate cancer cells.
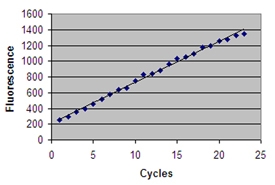
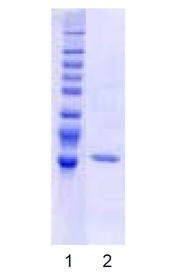
The full length USP2 protein is comprised of 618 amino acids with a predicted molecular mass of 69.4 kDa and contains the highly conserved Cys and His boxes present in all members of the UBP family of deubiquitinylating enzymes. The N-terminal region (residues 271-618) of USP2 contains all the necessary residues for catalysis and exhibits deubiquitinylating activity vs. ubiquitin-AMC (Prod. No. BML-SE211).
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?