Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.

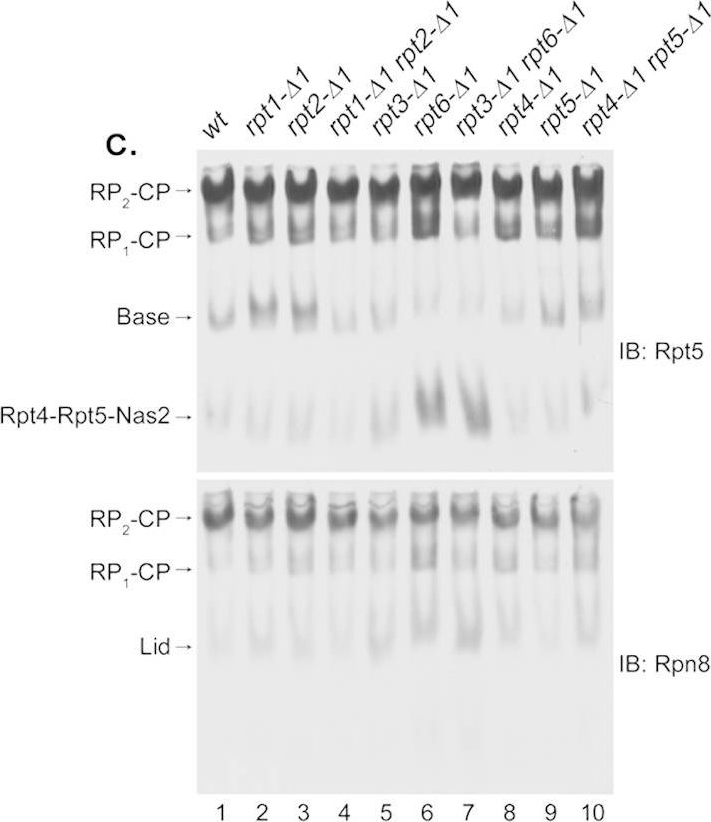
Nas6 maintains the abundance of RP species during Rpt tail-CP interaction.(a,b) Nas6 is not directly responsible for gate opening defects in the proteasome from rpt3-Δ1rpt6-Δ1 cells. Whole cell lysates (80 μg) were prepared in the presence of ATP (left) or ADP (right) at 2 mM each, and subjected to 3.5% native PAGE. In-gel peptidase assays with LLVY-AMC were performed to assess the gate opening in the proteasome holoenzymes in the absence or presence of 0.02% SDS. (c) Nas6 maintains intracellular levels of RP and base species. The native gels as in (a,b) were subjected to immunoblotting (IB) to detect proteasome holoenzymes and their subassemblies using an antibody to a base subunit Rpt5. RP species become prominent in rpt3-Δ1rpt6-Δ1 cells under ADP condition (tick mark, lanes 4, 12). (d,e) Proteasome activities (RP2-CP and RP1-CP) of the indicated strains from (a,b) were quantified using ImageJ and normalized to wild-type (% WT) as mean ± SEM (n = 6, ATP; n = 4, ADP). Calculations were performed individually for samples in the absence of SDS, and in the presence of 0.02% SDS. (f) Nas6 promotes RP dissociation from the proteasome in the presence of ADP. Proteasomes were purified with a Protein A tag appended to a lid subunit Rpn1144. Affinity-purified proteasomes (0.6 pmol) were incubated with 1, 5, and 25 fold molar excess of recombinant Nas6 for 30 min at 30 °C in the presence of ATP (2 mM) or ADP (2 mM). The samples were subjected to 3.5% native PAGE and immunoblotting for a base subunit Rpt5 and a lid subunit Rpn8 to detect proteasome holoenzymes and RP species. (g) Nas6 remains bound to the RP when RP-CP interaction is destabilized by ADP. RP was affinity-purified using Rpn11-TEV-ProA from the nas6Δ strain and was left on IgG resin. Immobilized nas6Δ RP (5 μl) was incubated with purified CP (3 μg) or recombinant Nas6 (0.5 μg), singly and in combination for 30 min at 30 °C, with either ATP or ADP (2 mM). RP-bound material was washed, eluted with TEV protease, and analyzed via 12% SDS-PAGE and immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)
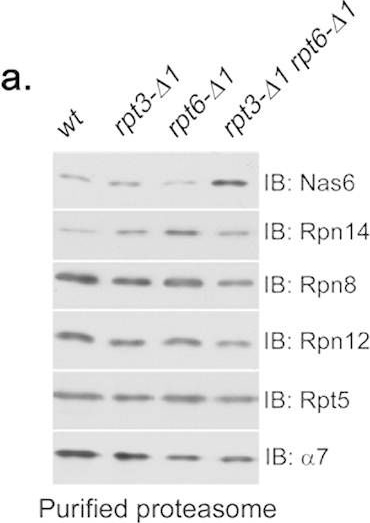
Rpt6 tail binding to the CP is required for Rpt3 tail-mediated release of Nas6 from the proteasome holoenzyme.(a) Increased retention of Nas6 in the proteasomes purified from rpt3-Δ1rpt6-Δ1 cells. Proteasomes were isolated from indicated strains via a Protein A tag that is appended to CP subunit Pre144. Purified proteasomes (1 μg) were subjected to 12% SDS-PAGE and immunoblotting (IB) for the indicated proteins: Nas6, Rpn14, chaperones; Rpn8, Rpn12, lid subunits; Rpt5, base subunit; α7, CP subunit. Pre1-TEV-ProA tag was chosen to exclude the purification of free RP. Note that Nas6 abundance remains unchanged in these cells (Fig. 1b). (b) Increased retention of Nas6 in the proteasomes in whole cell extracts from rpt3-Δ1rpt6-Δ1 cells. Whole cell lysates (40 μg) from indicated strains were resolved by 3.5% native gel (top panel). Following proteasome activity assay with LLVY-AMC, the native gel region containing the proteasome holoenzymes (RP2-CP and RP1-CP) was excised into a horizontal strip and directly subjected to 12% SDS-PAGE and immunoblotting for indicated proteins (bottom panel). Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. Note that decreased proteasome activities in rpt3-Δ1rpt6-Δ1 cells are due to gate opening defects (Fig. 2a,b). (c) Nas6 level in the proteasomes from rpt3-Δ1rpt4-Δ1 cells is comparable to that in the proteasomes from rpt3-Δ1 cells. Experiments were carried out as in (b) to assess the level of Nas6 in the proteasome holoenzymes from indicated cells. Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. (d) Phenotypic analysis of indicated yeast strains showing that the Rpt3 tail exhibits stronger genetic interaction with the Rpt6 tail than the Rpt4 tail. Four-fold serial dilutions of the indicated cells were spotted onto synthetic complete medium (SC) and grown for 2–3 days at 30 °C and 33 °C. For testing sensitivity to canavanine (an arginine analog), arginine was omitted from the SC medium. Canavanine was included at 1 μg/ml final concentration.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)
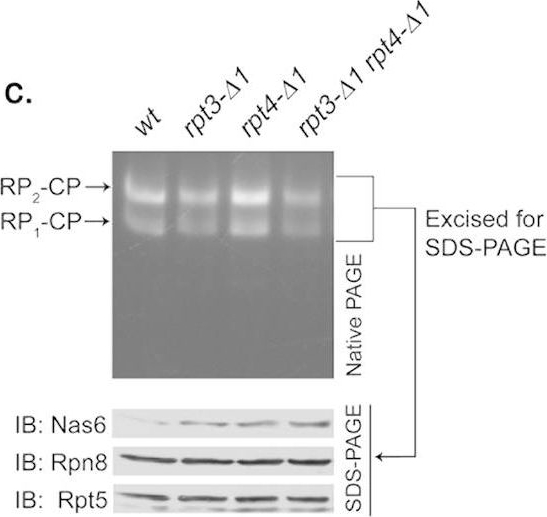
Rpt6 tail binding to the CP is required for Rpt3 tail-mediated release of Nas6 from the proteasome holoenzyme.(a) Increased retention of Nas6 in the proteasomes purified from rpt3-Δ1rpt6-Δ1 cells. Proteasomes were isolated from indicated strains via a Protein A tag that is appended to CP subunit Pre144. Purified proteasomes (1 μg) were subjected to 12% SDS-PAGE and immunoblotting (IB) for the indicated proteins: Nas6, Rpn14, chaperones; Rpn8, Rpn12, lid subunits; Rpt5, base subunit; α7, CP subunit. Pre1-TEV-ProA tag was chosen to exclude the purification of free RP. Note that Nas6 abundance remains unchanged in these cells (Fig. 1b). (b) Increased retention of Nas6 in the proteasomes in whole cell extracts from rpt3-Δ1rpt6-Δ1 cells. Whole cell lysates (40 μg) from indicated strains were resolved by 3.5% native gel (top panel). Following proteasome activity assay with LLVY-AMC, the native gel region containing the proteasome holoenzymes (RP2-CP and RP1-CP) was excised into a horizontal strip and directly subjected to 12% SDS-PAGE and immunoblotting for indicated proteins (bottom panel). Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. Note that decreased proteasome activities in rpt3-Δ1rpt6-Δ1 cells are due to gate opening defects (Fig. 2a,b). (c) Nas6 level in the proteasomes from rpt3-Δ1rpt4-Δ1 cells is comparable to that in the proteasomes from rpt3-Δ1 cells. Experiments were carried out as in (b) to assess the level of Nas6 in the proteasome holoenzymes from indicated cells. Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. (d) Phenotypic analysis of indicated yeast strains showing that the Rpt3 tail exhibits stronger genetic interaction with the Rpt6 tail than the Rpt4 tail. Four-fold serial dilutions of the indicated cells were spotted onto synthetic complete medium (SC) and grown for 2–3 days at 30 °C and 33 °C. For testing sensitivity to canavanine (an arginine analog), arginine was omitted from the SC medium. Canavanine was included at 1 μg/ml final concentration.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)
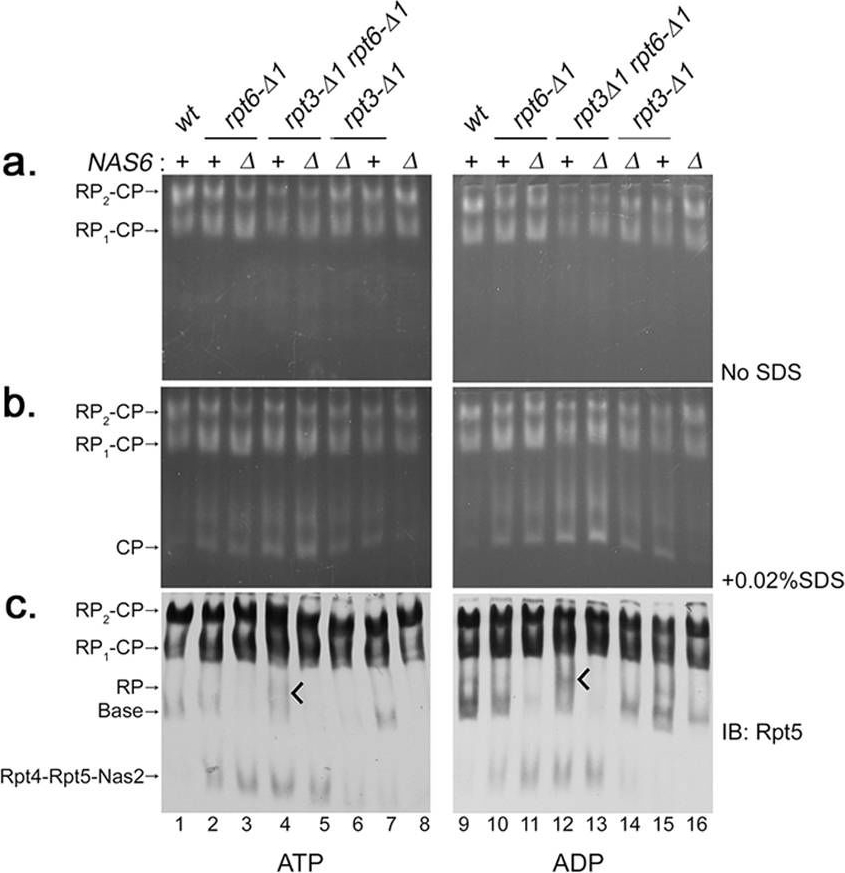
Nas6 maintains the abundance of RP species during Rpt tail-CP interaction.(a,b) Nas6 is not directly responsible for gate opening defects in the proteasome from rpt3-Δ1rpt6-Δ1 cells. Whole cell lysates (80 μg) were prepared in the presence of ATP (left) or ADP (right) at 2 mM each, and subjected to 3.5% native PAGE. In-gel peptidase assays with LLVY-AMC were performed to assess the gate opening in the proteasome holoenzymes in the absence or presence of 0.02% SDS. (c) Nas6 maintains intracellular levels of RP and base species. The native gels as in (a,b) were subjected to immunoblotting (IB) to detect proteasome holoenzymes and their subassemblies using an antibody to a base subunit Rpt5. RP species become prominent in rpt3-Δ1rpt6-Δ1 cells under ADP condition (tick mark, lanes 4, 12). (d,e) Proteasome activities (RP2-CP and RP1-CP) of the indicated strains from (a,b) were quantified using ImageJ and normalized to wild-type (% WT) as mean ± SEM (n = 6, ATP; n = 4, ADP). Calculations were performed individually for samples in the absence of SDS, and in the presence of 0.02% SDS. (f) Nas6 promotes RP dissociation from the proteasome in the presence of ADP. Proteasomes were purified with a Protein A tag appended to a lid subunit Rpn1144. Affinity-purified proteasomes (0.6 pmol) were incubated with 1, 5, and 25 fold molar excess of recombinant Nas6 for 30 min at 30 °C in the presence of ATP (2 mM) or ADP (2 mM). The samples were subjected to 3.5% native PAGE and immunoblotting for a base subunit Rpt5 and a lid subunit Rpn8 to detect proteasome holoenzymes and RP species. (g) Nas6 remains bound to the RP when RP-CP interaction is destabilized by ADP. RP was affinity-purified using Rpn11-TEV-ProA from the nas6Δ strain and was left on IgG resin. Immobilized nas6Δ RP (5 μl) was incubated with purified CP (3 μg) or recombinant Nas6 (0.5 μg), singly and in combination for 30 min at 30 °C, with either ATP or ADP (2 mM). RP-bound material was washed, eluted with TEV protease, and analyzed via 12% SDS-PAGE and immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)
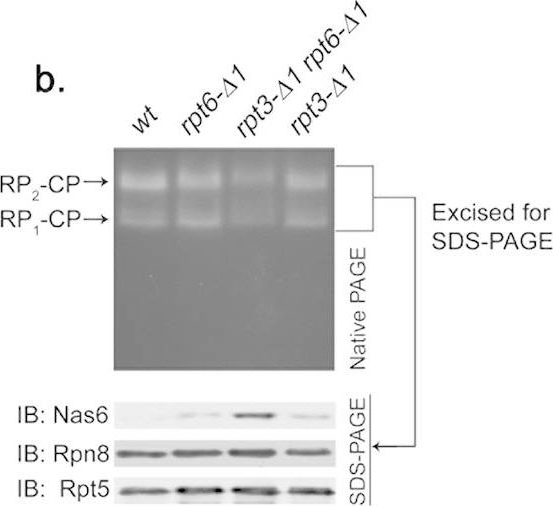
Rpt6 tail binding to the CP is required for Rpt3 tail-mediated release of Nas6 from the proteasome holoenzyme.(a) Increased retention of Nas6 in the proteasomes purified from rpt3-Δ1rpt6-Δ1 cells. Proteasomes were isolated from indicated strains via a Protein A tag that is appended to CP subunit Pre144. Purified proteasomes (1 μg) were subjected to 12% SDS-PAGE and immunoblotting (IB) for the indicated proteins: Nas6, Rpn14, chaperones; Rpn8, Rpn12, lid subunits; Rpt5, base subunit; α7, CP subunit. Pre1-TEV-ProA tag was chosen to exclude the purification of free RP. Note that Nas6 abundance remains unchanged in these cells (Fig. 1b). (b) Increased retention of Nas6 in the proteasomes in whole cell extracts from rpt3-Δ1rpt6-Δ1 cells. Whole cell lysates (40 μg) from indicated strains were resolved by 3.5% native gel (top panel). Following proteasome activity assay with LLVY-AMC, the native gel region containing the proteasome holoenzymes (RP2-CP and RP1-CP) was excised into a horizontal strip and directly subjected to 12% SDS-PAGE and immunoblotting for indicated proteins (bottom panel). Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. Note that decreased proteasome activities in rpt3-Δ1rpt6-Δ1 cells are due to gate opening defects (Fig. 2a,b). (c) Nas6 level in the proteasomes from rpt3-Δ1rpt4-Δ1 cells is comparable to that in the proteasomes from rpt3-Δ1 cells. Experiments were carried out as in (b) to assess the level of Nas6 in the proteasome holoenzymes from indicated cells. Rpn8 (a lid subunit) and Rpt5 (a base subunit) serve as loading controls for proteasome levels in the native gel strip. (d) Phenotypic analysis of indicated yeast strains showing that the Rpt3 tail exhibits stronger genetic interaction with the Rpt6 tail than the Rpt4 tail. Four-fold serial dilutions of the indicated cells were spotted onto synthetic complete medium (SC) and grown for 2–3 days at 30 °C and 33 °C. For testing sensitivity to canavanine (an arginine analog), arginine was omitted from the SC medium. Canavanine was included at 1 μg/ml final concentration.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)
Rpt6 tail binding to the CP is crucial for Rpt3 tail-mediated gate opening in the proteasome holoenzyme.(a) Decreased proteasome activity in rpt3-Δ1rpt6-Δ1 cells (top panel, RP2-CP and RP1-CP) is restored upon artificial opening of the CP gate (bottom panel, RP2-CP and RP1-CP). Whole cell lysates (80 μg) from indicated strains were resolved by 3.5% native PAGE. The native gel was incubated with fluorogenic peptide substrate LLVY-AMC to visualize the proteasomes (top panel). Following the imaging of the native gel, the same gel was further incubated with LLVY-AMC in the presence of 0.02% SDS (bottom panel). The addition of SDS is known to open the CP gate8. RP2-CP and RP1-CP are doubly-capped and singly-capped proteasome holoenzymes, respectively. The CP gate within wild-type proteasome holoenzymes is in an open configuration and is not further enhanced by 0.02% SDS8. (b) Quantification of relative proteasome activities (RP2-CP and RP1-CP) from seven independent experiments as in (a) plotted as mean ± standard error of the mean (SEM). Values of proteasome activities of the indicated strains were quantified using ImageJ software, and normalized to wild-type to obtain the relative proteasome activities. Calculations were performed individually for samples in the absence of SDS as in top panel from (a), and in the presence of 0.02% SDS as in bottom panel from (a) for each experiment. (c) Impaired RP assembly and defective proteasome holoenzymes in rpt3-Δ1rpt6-Δ1 cells. Following proteasome activity assays with the LLVY-AMC as in (a), the native gels were subjected to immunoblotting (IB) to detect the proteasome holoenzymes and their subassemblies using antibodies to a base subunit Rpt5 (top panel), and a lid subunit Rpn8 (bottom panel).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly. Sci Rep (2015)






Product Details
| Alternative Name |
26S protease regulatory subunit 6A, Tat-binding protein homolog 1, TBP-1 |
|---|---|
| Application |
WB |
| Formulation |
Liquid. In PBS containing 10mM sodium azide. |
| GenBank ID |
464859 |
| Host |
Rabbit |
| Immunogen |
Recombinant protein corresponding to the N-terminal 100 amino acids of Yta1. |
| Species Reactivity |
Yeast |
| Specificity |
Recognizes the Rpt5/S6a subunit of the 19S regulator complex. |
| UniProt ID |
P33297 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Long Term Storage |
-20°C |
|---|---|
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Assembly chaperone Nas6 selectively destabilizes 26S proteasomes with defective regulatory particle-core particle interfaces: J.L. Warnock, et al.; J. Biol. Chem. 299, 102894 (2023), Abstract
- Assembly checkpoint of the proteasome regulatory particle is activated by coordinated actions of proteasomal ATPase chaperones: A. Nahar, et al.; Cell Rep. 39, 110918 (2022), Reactant(s) Saccharomyces cerevisiae, Abstract
- DET1-mediated COP1 regulation avoids HY5 activity over second-site gene targets to tune plant photomorphogenesis: Canibano, E., Bourbousse, C., et al.; Mol. Plant 14, 963 (2021), Abstract
- DET1-mediated COP1 regulation avoids HY5 activity over second-site targets to tune plant photomorphogenesis: Canibano, E., Bourbousse, C., et al.; bioRxiv , (2020)
- Proteasome subunit α1 overexpression preferentially drives canonical proteasome biogenesis and enhances stress tolerance in yeast: L.A. Howell, et al.; Sci. Rep. 9, 12418 (2019), Abstract — Full Text
- DET1-mediated degradation of a SAGA-like deubiquitination module controls H2Bub homeostasis: A. Nassrallah, et al.; Elife 7, e37892 (2018), Application(s): WB, Abstract — Full Text
- Molecular bases for the constitutive photomorphogenic phenotypes in Arabidopsis.: Huq, E., Xu, X., et al.; Development 145, (2018), Application(s): WB, Abstract
- Molecular bases for the constitutive photomorphogenic phenotypes in i>Arabidopsis/i>: Huq, E., Huq, E., et al.; bioRxiv , (2018), Application(s): WB
- Dynamic regulation of PIF5 by COP1-SPA complex to optimize photomorphogenesis in Arabidopsis: Pham, V. N., Kathare, P. K., et al.; Plant J. 96, 260 (2018), Abstract
- Cross-species complementation reveals conserved functions for EARLY FLOWERING 3 between monocots and dicots.: Huang, H., Gehan, M. A., et al.; Plant Direct. 1, e00018 (2017), Application(s): WB / Reactant(s): Saccharomyces cerevisiae, Abstract
- Cross-species complementation reveals conserved functions for EARLY FLOWERING 3 between monocots and dicots: Huang, H., Alvarez, S., et al.; bioRxiv , (2017), Application(s): WB / Reactant(s): Saccharomyces cerevisiae
- Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation: W.H. Choi, et al.; Nat. Commun. 7, 10963 (2016), Application(s): Western blot, Abstract
- An evolutionarily conserved pathway controls proteasome homeostasis: A. Rousseau, et al.; Nature 536, 184 (2016), Application(s): WB, Abstract — Full Text
- The F-box Protein Rcy1 Is Involved in the Degradation of Histone H3 Variant Cse4 and Genome Maintenance.: Rao, H., Bao, X., et al.; J. Biol. Chem. 291, 10372 (2016), Application(s): WB / Reactant(s): Saccharomyces cerevisiae, Abstract
- Proteasome Activation is Mediated via a Functional Switch of the Rpt6 C-terminal Tail Following Chaperone-dependent Assembly: V. Sokolova, et al.; Sci. Rep. 5, 14909 (2015), Application(s): WB / Reactant(s) Saccharomyces cerevisiae, Abstract — Full Text
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
