HDAC inhibitor
Tubacin (tubulin acetylation inducer) is a highly potent and selective, reversible, cell-permeable inhibitor of HDAC6 (IC50=0.004µM). IC50‘s for the other HDACs are 1000-fold higher, making tubacin both more selective and more potent than Tubastatin A, which also inhibits HDAC8. Concentration in cell culture experiments typically ranges from 2-50µM.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
Product Details
| Alternative Name |
N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-1,3-oxazol-2-yl)sulfanyl]methyl}-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl}phenyl)-N’-hydroxyoctanediamide |
|---|---|
| Appearance |
White solid. |
| CAS |
537049-40-4 |
| Couple Target |
HDAC, HDAC6 |
| Couple Type |
Inhibitor |
| Formula |
C41H43N3O7S |
| MW |
721.9 |
| Purity |
≥96% (ELSD) |
| Solubility |
Soluble in DMSO (10 mg/ml). |
| Source |
Synthetic. |
Handling & Storage
| Use/Stability |
As indicated on product label or CoA when stored as recommended. Stable for at least 1 year after receipt when stored at -80°C. |
|---|---|
| Short Term Storage |
-20°C |
| Long Term Storage |
-80°C |
| Shipping |
Dry Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- A live-cell marker to visualize the dynamics of stable microtubules throughout the cell cycle: K.I. Jansen, et al.; J. Cell Biol. 222, e202106105 (2023), Abstract
- Histone deacetylase-6 modulates the effects of 4°C platelets on vascular endothelial permeability: B. Miyazawa, et al.; Blood Adv. 7, 1241 (2023), Abstract
- Inhibition of HDAC6 Activity Protects Against Endothelial Dysfunction and Atherogenesis in vivo: A Role for HDAC6 Neddylation: Y. Nomura, et al.; Front. Physiol. 12, 675724 (2021), Abstract
- HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation: Magupalli, V. G., Negro, R., et al.; Science 369, (2020), Abstract
- LC3A Silencing Hinders Aggresome Vimentin Cage Clearance in Primary Choroid Plexus Carcinoma: M. Nassar, et al.; Sci. Rep. 7, 8022 (2017), Application(s): Measurement of cell index against different concentration, Abstract — Full Text
- Neurotoxic mechanisms of paclitaxel are local to the distal axon and independent of transport defects: Gornstein, E. L., Schwarz, T. L., et al.; Exp. Neurol. 288, 153 (2017), Abstract
- Limited efficacy of specific HDAC6 inhibition in urothelial cancer cells: Rosik, L., Niegisch, G., et al.; Cancer Biol. Ther. 15, 742 (2014), Abstract
- Tenovin-D3, a novel small-molecule inhibitor of sirtuin SirT2, increases p21 (CDKN1A) expression in a p53-independent manner: McCarthy, A. R., Sachweh, M. C., et al.; Mol. Cancer Ther. 12, 352 (2013), Abstract
- Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, Tubastatin A: K.V. Butler, et al.; J. Am. Chem. Soc. 370, 10842 (2010), Abstract
- Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents: M. Namdar, et al.; Proc. Natl. Acad. Sci. USA 107, 20003 (2010), Abstract — Full Text
- Tubacin kills Epstein-Barr virus (EBV)-Burkitt lymphoma cells by inducing reactive oxygen species and EBV lymphoblastoid cells by inducing apoptosis: J. Kawada, et al.; J. Biol. Chem. 284, 17102 (2009), Abstract — Full Text
- Histone deacetylase 6 interacts with the microtubule-associated protein tau: H. Ding, et al.; J. Neurochem. 106, 2119 (2008), Abstract — Full Text
- Structural biasing elements for in-cell histone deacetylase paralog selectivity: J.C. Wong, et al.; J. Am. Chem. Soc. 125, 5586 (2003), Abstract
- Multidimensional chemical genetic analysis of diversity-oriented synthesis-derived deacetylase inhibitors using cell-based assays: S.J. Haggarty, et al.; Chem. Biol. 10, 383 (2003), Abstract
- Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation: S.J. Haggarty, et al.; Proc. Natl. Acad. Sci. USA 100, 4389 (2003), Abstract — Full Text
- Synthesis of 7200 small molecules based on a substructural analysis of the histone deacetylase inhibitors trichostatin and trapoxin: S.M. Sternson, et al.; Org. Lett. 3, 4239 (2001), Abstract
Related Products

| Application | Activity assay, Fluorescent detection, HTS |
|---|
| Alternative Name | Histone deacetylase 6 |
|---|---|
| Source | Produced in insect cells. Produced in a baculovirus expression system. |
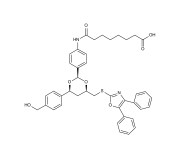
| Alternative Name | 7-(4-((2R,4R,6S)-4-((4,5-dipenyloxazol-2-ylthio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenylcarbamoyl)heptanoic acid |
|---|---|
| Couple Type | Inhibitor |
| Purity | ≥98% (HPLC) |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?