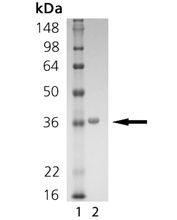
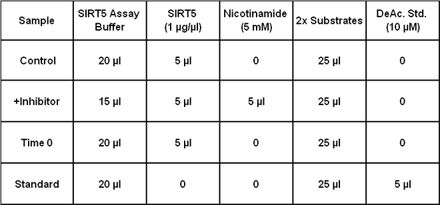
SIRT5, along with SIRTs 3 and 4, is one of three human mitochondrial sirtuins (homologs of yeast Sir2). SIRT5, which is localized to both the mitochondrial matrix and the intermembrane space, is an NAD+-dependent lysine deacetylase (class III HDAC). SIRT5’s only known physiological target is the intermembrane space protein, cytochrome c, suggesting the possibility of regulatory roles in metabolism and/or apoptosis. SIRT5 has also been shown to deacetylate and thereby upregulate the matrix urea cycle enzyme carbamoyl phosphate synthetase 1. This implies an important role for SIRT5 in ammonia detoxification and adaptation to elevated amino acid catabolism during starvation, calorie restriction or a high protein diet. SIRT5 is most strongly expressed in thymus, lymphoblasts and heart muscle cells and its chromosomal location (6p23) has been implicated in chromosomal abnormalities associated with malignancies. SIRT5 deacetylase activity may be measured with the FLUOR DE LYS® Desuccinylase (Prod. No. BML-KI590) or SIRT1 (Prod. No. BML-KI177) substrates, and, like SIRT1, its activity is stimulated by polyphenols such as resveratrol and fisetin. Two SIRT5 crystal structures have been determined, one as a complex with ADP-ribose and the other a complex with the inhibitor suramin. This recombinant preparation, expressed in E. coli, comprises residues 37-310 of SIRT5 isoform 1, with an N-terminal His-tag. Recombinant human mitochondrial processing protease cleaves full-length SIRT5 after residue thirty-six, suggesting that the mature, in vivo form may begin with residue 37. Consistent with this, N-terminal sequence of mouse SIRT5 processed in 293T cells also commences at residue 37.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?