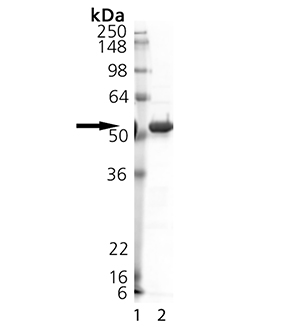
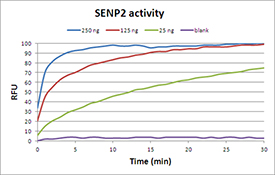
Post-translation modification of proteins plays a crucial role in altering protein function, localization, and turnover. Modification includes not only chemical modifications such as acetylation and phosphorylation but also the conjugation of proteins to target substrates. Although ubiquitylation is generally regarded to target a protein for degradation, it is now clear that certain types of ubiquitin modifications may have other regulatory effects. The covalent modification of proteins by SUMO-1 is reversible and is mediated by SUMO-specific proteases. These proteases are thought to have dual roles. They are responsible first for the initial processing of SUMO-1 by cleavage of the precursor peptide at the carboxyl terminus of the protein, generating a C-terminal diglycine motif, and second for the subsequent processing and cleavage of high molecular weight SUMO-1 conjugates, releasing SUMO-1 and reducing the conjugation status of the target proteins. A number of SUMO-specific proteases have been predicted based on homology to the first identified protease yeast Ulp1, the Smt3 (SUMO-1 homologue) deconjugating enzyme, and are thought to be members of the general family of cysteine proteases. SENP2 was discovered both through its homology to other SUMO proteases and its interactions with murine Axin, a regulator of the Wnt signalling pathway. When overexpressed in tissue culture cells or under in vitro conditions, the murine SENP2 homologue (Smt3IP2) cleaves conjugates of SUMO-1, SUMO-2, and SUMO-3. It has been shown that full-length human SENP2 associates with nuclear pores in a manner similar to Ulp1 in yeast. This association occurs exclusively with the nuclear face of the pore and requires sequences near the N-terminus of SENP2. SENP2 also binds specifically to Nup153, a nucleoporin localized to the nucleoplasmic face of the nuclear pore and this association requires the same domain of SENP2 that mediates its targeting in vivo. A mutant SENP2 protein that is unable to bind Nup153 is significantly more effective in promoting deconjugation of SUMO-1-conjugated species, indicating that localization of SENP2 to the nuclear pore plays an important role in spatially restricting the activity of this enzyme.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?