Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
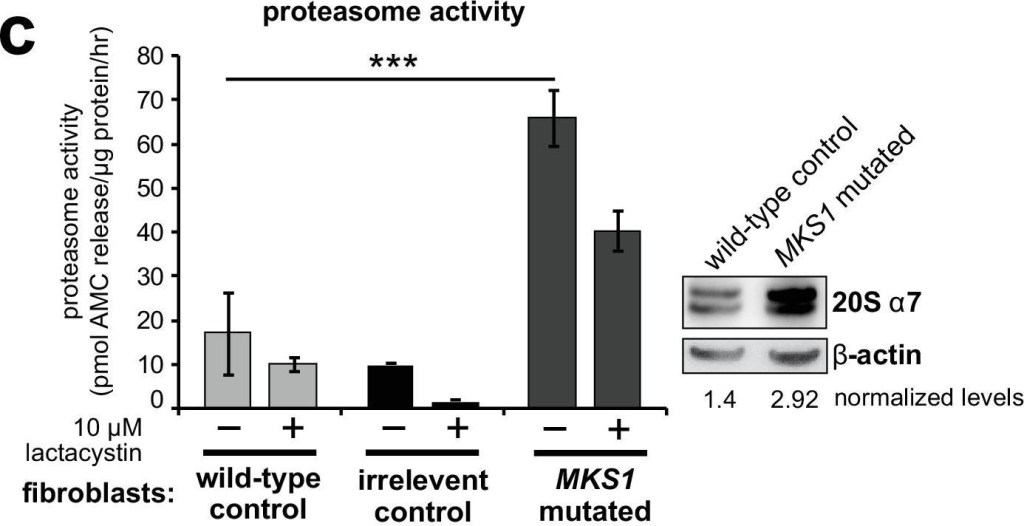
Deregulation of canonical Wnt signalling and proteasome activity following loss or mutation of MKS1.(a) Immunoblots for total soluble β-catenin, phospho-β-catenin, cyclin D1 and β-actin (loading control) in either wild-type normal or MKS1-mutated immortalised human fibroblasts from an MKS patient (MKS-562) following treatment with MG-132 proteasome inhibitor (+) or vehicle control (-). (b) SUPER-TOPFlash assays of canonical Wnt signalling activity in human MKS1-mutated fibroblasts compared to wild-type control fibroblasts following treatment with control conditioned medium, Wnt5a, Wnt3a, or a mixture of Wnt3a and Wnt5a media, as indicated. Statistical significance of pairwise comparisons is shown (* indicates p < 0.05, paired two-tailed Student t-test). Error bars indicate s.e.m. with results shown for four independent biological replicates. (c) Proteasome activity assays for wild-type or MKS1-mutated human fibroblasts or an irrelevant control (ASPM-mutant fibroblasts), following treatment with c-lactacystin-β-lactone (+) or vehicle control (-). Statistical significance of pairwise comparison as for (b); *** indicates p < 0.001 for three independent biological replicates. Immunoblots show levels of the 20 S proteasome α7 subunit compared to β-actin loading control. (d) Protease activity assays of crude proteasome preparations from Mks1+/+ or Mks1-/- mouse embryonic fibroblasts (MEFs), expressed as pmol AMC released per µg proteasome per hr. Treatment with lactacystin is the assay control. Statistical analysis as for (b); ** indicates p T]+[IVS15-7_35del29] causing the predicted nonsense and splice-site mutations [p.R158*]+[p.P470fs*562]. Additional smaller PCR products in MKS1 patient corresponds to skipping of exon 5 and exon 16, confirmed by Sanger sequencing, due to the frameshift mutation affecting splicing. (b) Immunoblot showing loss of MKS1 protein in MKS1-mutated patient fibroblasts compared to healthy controls; loading control is β-actin. (c) IF microscopy images of wild-type control and MKS1-mutated fibroblasts showing loss of cilia and disorganisation of cytoskeleton in patient cells. Bar graphs quantify reductions in incidence and length of cilia in patient cells. Statistical significance of pairwise comparisons with control for three independent biological replicates are shown (*** p < 0.001, **** p < 0.0001; paired two-tailed Student t-test; error bars indicate s.e.m.) (d) IF microscopy images showing loss of MKS1 ciliary localisation in MKS1-mutated patient fibroblasts compared to wild-type control fibroblasts (indicated by arrowheads). (e) Western blot showing increased levels of polyubiquitinated protein in MKS1-mutated fibroblasts after MG-132 proteasome inhibition. and shUbe2e1 after MG-132 treatment.Figure 1—figure supplement 1—source data 1.Characterisation of MKS1-mutated human patient fibroblasts: full western blots & gels.Characterisation of MKS1-mutated human patient fibroblasts: full western blots & gels.In vivo loss of MKS1 causes deregulated ubiquitin-proteasome processing.Proteasome defects (GFP; green) in Mks1-/- x UbG76V-GFP E12.5 embryonic heart (top panels) and liver (middle panels) as indicated, with accumulation of active β-catenin (red) and GFP-tagged ubiquitin (green) in the neuroepithelium of E12.5 Mks1-/-xUbG76VGFP embryonic neocortex. Scale bars = 50 μm. White frames indicate magnified regions displayed in insets.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Regulation of canonical Wnt signalling by the ciliopathy protein MKS1 and the E2 ubiquitin-conjugating enzyme UBE2E1. Elife (2022)
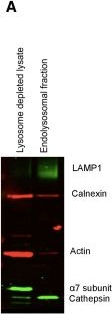
Differential centrifugation of PBMC lysate yields fractions that are highly enriched in endolysosomes. (A) Enrichment of endolysosomes in fractions recovered after differential centrifugation was assessed by western blot. PBMC lysate from which endolysosomes were extracted (lane 1), fraction enriched in endolysosomal proteins LAMP1 and Cathepsin S (lane 2). (B) Isolated endolysosomal (light grey) and cytosol depleted of lysosomal (dark grey) fractions were assayed for activity of cathepsins, aminopeptidases and chymotryptic activity of proteasome in the presence of inhibitors Epoxomicin (Epox) for proteasome, Bestatin (Best) for aminopeptidase, E64 for cathepsins and EDTA as a metal chelator. Mean and SD of replicates from two different donors are plotted. (C) Acid phosphatase assay was performed to confirm enrichment of endolysosomes in different fractions. Figure representative of acid phosphatase activities in one fractionation experiment done in triplicates. Mean and SD are plotted. (D) Two HIV Gag peptides, B57KF11 (KAFSPEVIPMF) and B27KK10 (KRWIILGLNK) were subjected to degradation by equivalent amounts of purified cytosol (blue) or endolysosomes (red). An aliquot of the reaction mixture was stopped at periodic intervals and the percentage of original peptide remaining was determined by HPLC. The half-life of the peptide in both conditions was calculated (36 min in cytosol and 8 min in endolysosomes for B57KF11 and more than 60 min in cytosol and 38 min in endolysosome for B27KK10). Figure 1D is representative of two replicates.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: A simple methodology to assess endolysosomal protease activity involved in antigen processing in human primary cells. BMC Cell Biol (2013)


Product Details
| Alternative Name |
Proteasome subunit α type-3, Macropain subunit C8 |
|---|---|
| Application |
IHC, WB |
| Clone |
MCP72 |
| Formulation |
Liquid. In PBS containing 10mM sodium azide. |
| Gene/Protein Identifier |
PSMA3 (gene name) |
| Host |
Mouse |
| Immunogen |
Dinitrophenylated human placenta derived proteasomes. |
| Isotype |
IgG1 |
| Purity Detail |
Partially purified ascites. |
| Source |
Purified from hybridoma tissue culture supernatant. |
| Species Reactivity |
Arthropod, Human, Rabbit, Rat, Yeast |
| Specificity |
Recognizes the α7 subunit of the 20S proteasome. |
| Technical Info / Product Notes |
Various systems for the nomenclature of the proteasome subunits have been established. This may be a source of confusion as the system on UniProt differs from “standard” nomenclature as described in the literature. The UniProt ID and Gene Name will help to clearly identify the proteins. |
| UniProt ID |
P25788 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. After opening, prepare aliquots and store at -20°C. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- A chaperone-proteasome-based fragmentation machinery is essential for aggrephagy.: Mauthe, M., van de Beek, N., et al.; Nat. Cell Biol. 27, 1448 (2025), Abstract
- The proteasome maturation factor POMP moonlights as a stress-induced transcriptional regulator: Giandomenico, S. L., Mueller, M., et al.; bioRxiv , (2025), Application(s): Immunofluorescence, Western blot
- Temporal control of acute protein aggregate turnover by UBE3C and NRF1-dependent proteasomal pathways.: Hickey, K. L., Panov, A., et al.; PNAS 121, e2417390121 (2024), Abstract
- The proteomic landscape of synaptic diversity across brain regions and cell types: van Oostrum, M., Blok, T. M., et al.; Cell 186, 5411 (2023), Abstract
- TRIM25 targets p300 for degradation: Elabd, S., Pauletto, E., et al.; Life Sci. Alliance 6, (2023), Abstract
- Regulation of canonical Wnt signalling by the ciliopathy protein MKS1 and the E2 ubiquitin-conjugating enzyme UBE2E1.: Boldt, K., Ueffing, M., et al.; Elife 11, (2022), Application(s): WB / Reactant(s): Human, Abstract
- Antioxidant and Antiaging Properties of a Novel Synergistic Nutraceutical Complex: Readouts from an In Cellulo Study and an In Vivo Prospective, Randomized Trial.: Athanasopoulou, S., Kapetanou, M., et al.; Antioxidants (Basel) 11, (2022), Application(s): WB / Reactant(s): Human, Abstract
- Reduction in PA28αβ activation in HD mouse brain correlates to increased mHTT aggregation in cell models: K.W. Geijtenbeek, et al.; PLoS One 17, e0278130 (2022), Abstract
- Healthspan improvement and anti-aggregation effects induced by a marine-derived structural proteasome activator: M.A. Vasilopoulou, et al.; Redox Biol. 56, 10262 (2022), Abstract
- Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice: A. Salazar-Degracia, et al.; J. Cell. Physiol. 234, 18041 (2019), Abstract
- Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia: A. Salazar-Degracia, et al.; Biochimie 149, 79 (2018), Abstract
- Involvement of the ubiquitin-proteasome system in the expression of extracellular matrix genes in retinal pigment epithelial cells: J.E. Ramos de Carvalho, et al.; Biochem. Biophys. Rep. 13, 83 (2018), Application(s): WB / Reactant(s) Human, Abstract
- Combined treatment of human multiple myeloma cells with bortezomib and doxorubicin alters the interactome of 20S proteasomes.: Fedorova, O. A., Kuzyk, V. O., et al.; Cell Cycle 17, 1745 (2018), Application(s): WB / Reactant(s): Human, Abstract
- Docosahexaenoic acid-mediated protein aggregates may reduce proteasome activity and delay myotube degradation during muscle atrophy in vitro: S.K. Shin, et al.; Exp. Mol. Med. 49, e287 (2017), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- Proteasome activation enhances stemness and lifespan of human mesenchymal stem cells: M. Kapetanou, et al.; Free Radic. Biol. Med. 103, 226 (2017), Abstract
- Modulation of the Proteasome Pathway by Nano-Curcumin and Curcumin in Retinal Pigment Epithelial Cells.: Reits, E. A., Schlingemann, R. O., et al.; Ophthalmic Res. 59, 98 (2017), Application(s): WB / Reactant(s): Human, Abstract
- Denervation-Induced Activation of the Ubiquitin-Proteasome System Reduces Skeletal Muscle Quantity Not Quality: C.W. Baumann, et al.; PLoS One 11, e0160839 (2016), Application(s): Western blot, left soleus muscles, Abstract — Full Text
- Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation: Itakura, E., Zavodszky, E., et al.; Mol. Cell 63, 21 (2016), Abstract
- Inhibition of Proteasome Activity Induces Formation of Alternative Proteasome Complexes.: Eickelberg, O., Trümbach, D., et al.; J. Biol. Chem. 291, 13147 (2016), Application(s): WB / Reactant(s): Human, Abstract
- Time-Course of Muscle Mass Loss, Damage, and Proteolysis in Gastrocnemius following Unloading and Reloading: Implications in Chronic Diseases: A. Chacon-Cabrera, et al.; PLoS One 11, e0164951 (2016), Application(s): Immunoblotting of 1D electrophoresis, frozen mouse muscle samples, Abstract — Full Text
- Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation: W.H. Choi, et al.; Nat. Commun. 7, 10963 (2016), Application(s): Western blot, Abstract
- Bortezomib Amplifies Effect on Intracellular Proteasomes by Changing Proteasome Structure: D.S. Pitcher, et al.; EBioMedicine 2, 642 (2015), Application(s): Western Blot, Abstract
- p53 is active in murine stem cells and alters the transcriptome in a manner that is reminiscent of mutant p53: H. Yan, et al.; Cell Death Dis. 6, e1662 (2015), Application(s): Western Blotting, Abstract — Full Text
- The proteasome function reporter GFPu accumulates in young brains of the APPswe/PS1dE9 Alzheimer’s disease mouse model.: Zhang, D., Rezvani, K., et al.; Cell. Mol. Neurobiol. 34, 315 (2014), Application(s): WB, Abstract
- Dysregulation of ubiquitin homeostasis and β-catenin signaling promote spinal muscular atrophy: Wishart, T. M., Mutsaers, C. A., et al.; J. Clin. Invest. 124, 1821 (2014), Abstract
- Nuclear proteasomes carry a constitutive posttranslational modification which derails SDS-PAGE (but not CTAB-PAGE): D.S. Pitcher, et al.; Biochim. Biophys. Acta 1844, 2222 (2014), Application(s): WB / Reactant(s) E. coli, Abstract
- A simple methodology to assess endolysosomal protease activity involved in antigen processing in human primary cells.: Xu, Y., Vaithilingam, A., et al.; BMC Cell Biol. 14, 35 (2013), Application(s): WB / Reactant(s): Human, Abstract
- TRIM5α associates with proteasomal subunits in cells while in complex with HIV-1 virions: Z. Lukic, et al.; Retrovirology 8, 93 (2011), Abstract — Full Text
Related Products
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?