Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
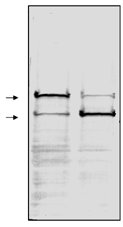
Western blot analysis of whole cell extracts from (1) human HL60 leukemia cells (Prod. No. BML-SW101) and (2) HL60 cells induced to undergo apoptosis using etoposide (Prod. No. BML-SW102). The nitrocellulose membrane was probed with purified clone C-2-10 mouse monoclonal anti-PARP (Prod. No. BML-SA249) at 1 µg/ml. Secondary antibody was GAM-AP (1/2000) and the nitrocellulose membrane was developed with BCIP/NBT. Arrows correspond to 116kDa and 85kDa.
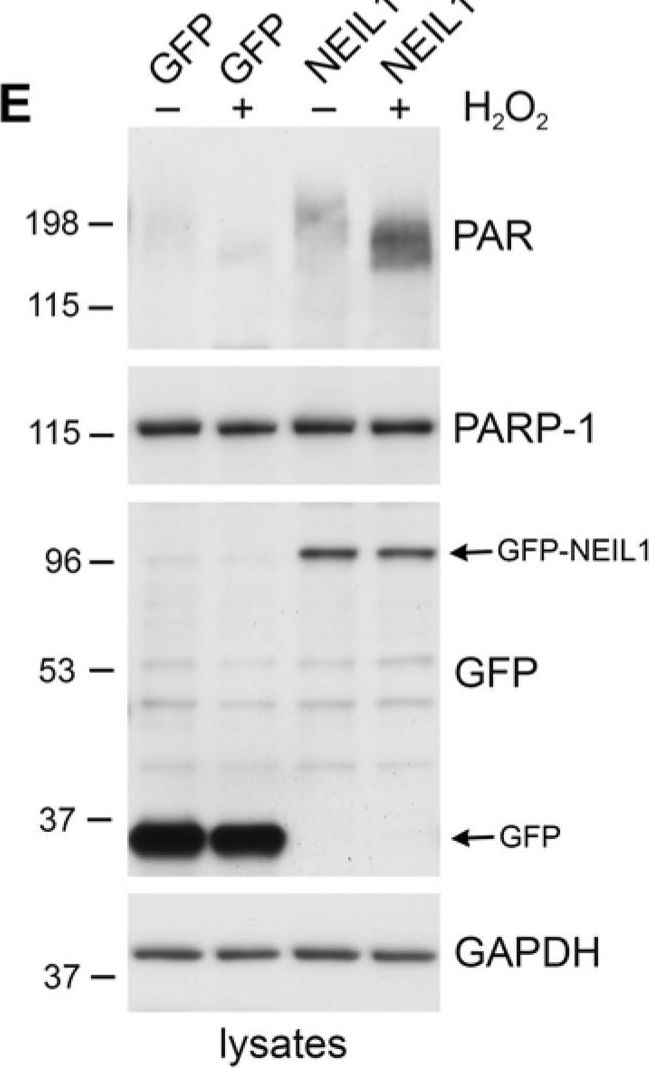
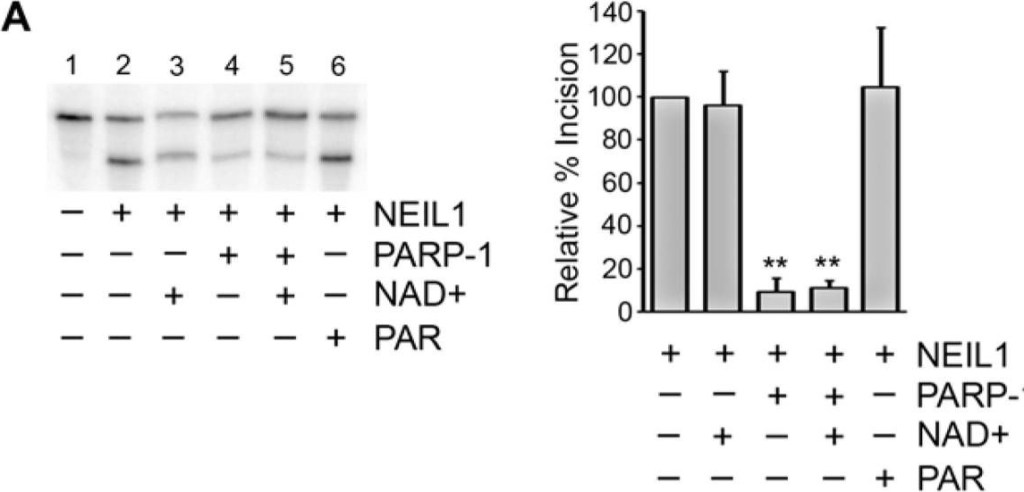
NEIL1 stimulates the poly(ADP-ribosyl)ation activity of PARP-1(A) PARP-1 activity was measured by determining the amount of PAR deposited on immobilized histones in an ELISA assay. Addition of NEIL1 (33 ng) increased the amount of PAR synthesis by PARP-1 (2 ng). The histogram represents the mean + SEM from three independent experiments. *p < 0.05 compared to control by Student's t-test. (B,C) GST, GST-NEIL1, GST-NEIL1 1–288 aa or GST-NEIL1 289–390 aa (5 μg) were incubated with (+) or without (−) 7.5 ng PARP-1. The immunoblot was stained with Ponceau S and probed with anti-PAR antibodies. (D) WT or NEIL1−/− MEFs were mock treated (−) or treated (+) with 500 μM H2O2. Lysates were probed with anti-PAR antibodies and reprobed with anti-PARP-1 and anti-GAPDH antibodies as a protein loading control. (E) NEIL1−/− MEFs transfected with pEGFP-NEIL1 or pEGFP control were mock treated (−) or treated (+) with 500 μM H2O2 and lysates were probed as above. Anti-GFP antibodies were used to examine levels of the transfected proteins. For (B-E), a representative experiment from at least 3 independent experiments is shown.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
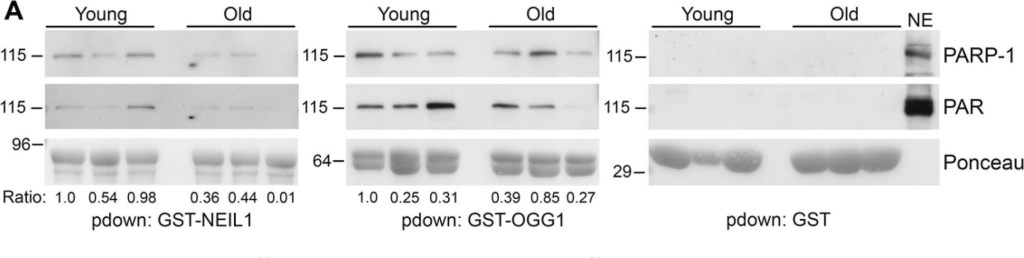
Decreased binding of PARP-1 to GST-NEIL1 with mouse age(A) Liver nuclear extracts were made from 3 different young (7 month) and 3 different old (17 month) mice and used for GST-precipitations with the indicated fusion proteins. Precipitations were probed with anti-PARP-1 and anti-PAR antibodies and stained with Ponceau S to visualize the fusion proteins. The relative amount of PARP-1 binding and PAR levels in precipitations were quantified from immunoblots and normalized to the amount of GST-fusion protein. The numbers below the blots represent the relative level of PARP-1 binding and the histograms in (B) represent the average PARP-1 binding and PAR levels in precipitations from the 3 young and 3 old mice. *P<0.05 comparing young and old mice using Student's t-test. Similar results were obtained in another independent experiment. (C) Liver nuclear extracts from young and old mice were probed by immunoblotting with anti-PARP-1 and anti-NEIL1 antibodies and reprobed with anti-TBP antibodies as a protein loading control. The numbers below the blots represent the level of PARP-1 or NEIL1 normalized to TBP. The histogram in (D) represents the average levels of NEIL1 and PARP-1 + SEM from the 3 young and 3 old mice.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
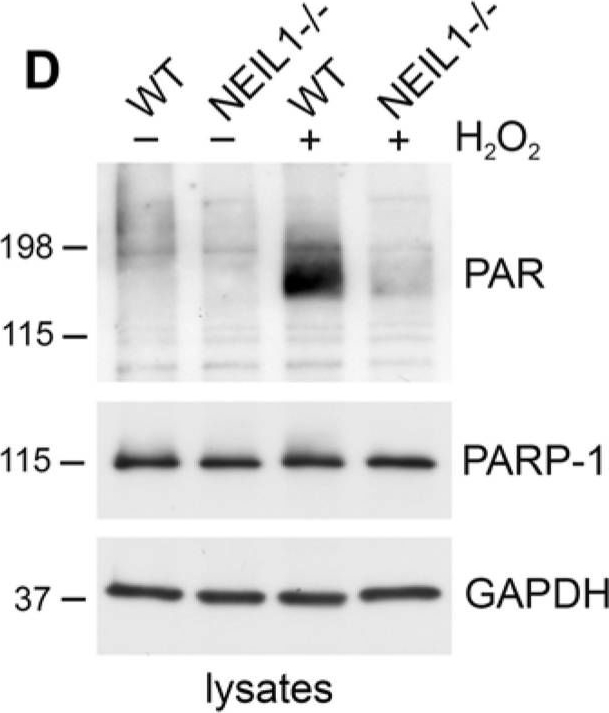
NEIL1 stimulates the poly(ADP-ribosyl)ation activity of PARP-1(A) PARP-1 activity was measured by determining the amount of PAR deposited on immobilized histones in an ELISA assay. Addition of NEIL1 (33 ng) increased the amount of PAR synthesis by PARP-1 (2 ng). The histogram represents the mean + SEM from three independent experiments. *p < 0.05 compared to control by Student's t-test. (B,C) GST, GST-NEIL1, GST-NEIL1 1–288 aa or GST-NEIL1 289–390 aa (5 μg) were incubated with (+) or without (−) 7.5 ng PARP-1. The immunoblot was stained with Ponceau S and probed with anti-PAR antibodies. (D) WT or NEIL1−/− MEFs were mock treated (−) or treated (+) with 500 μM H2O2. Lysates were probed with anti-PAR antibodies and reprobed with anti-PARP-1 and anti-GAPDH antibodies as a protein loading control. (E) NEIL1−/− MEFs transfected with pEGFP-NEIL1 or pEGFP control were mock treated (−) or treated (+) with 500 μM H2O2 and lysates were probed as above. Anti-GFP antibodies were used to examine levels of the transfected proteins. For (B-E), a representative experiment from at least 3 independent experiments is shown.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
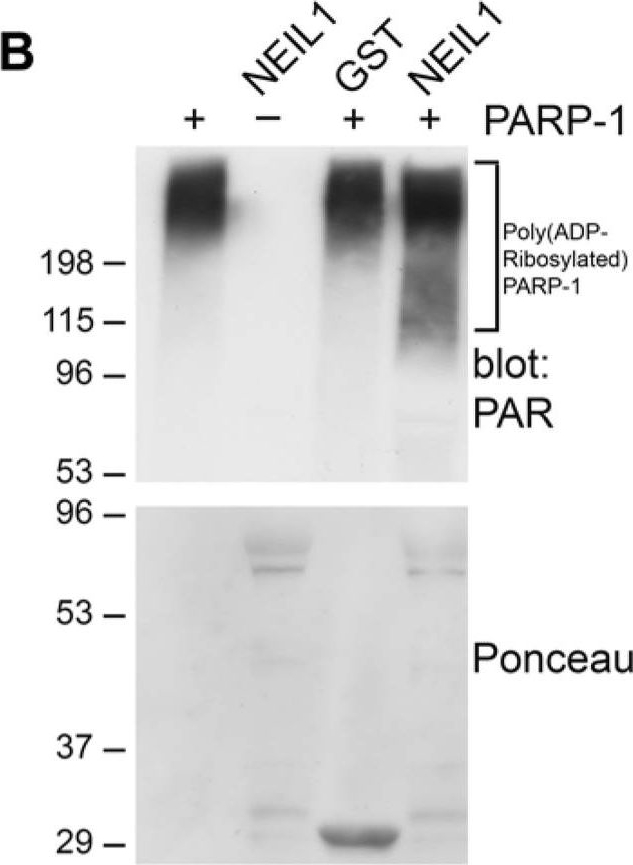
NEIL1 stimulates the poly(ADP-ribosyl)ation activity of PARP-1(A) PARP-1 activity was measured by determining the amount of PAR deposited on immobilized histones in an ELISA assay. Addition of NEIL1 (33 ng) increased the amount of PAR synthesis by PARP-1 (2 ng). The histogram represents the mean + SEM from three independent experiments. *p < 0.05 compared to control by Student's t-test. (B,C) GST, GST-NEIL1, GST-NEIL1 1–288 aa or GST-NEIL1 289–390 aa (5 μg) were incubated with (+) or without (−) 7.5 ng PARP-1. The immunoblot was stained with Ponceau S and probed with anti-PAR antibodies. (D) WT or NEIL1−/− MEFs were mock treated (−) or treated (+) with 500 μM H2O2. Lysates were probed with anti-PAR antibodies and reprobed with anti-PARP-1 and anti-GAPDH antibodies as a protein loading control. (E) NEIL1−/− MEFs transfected with pEGFP-NEIL1 or pEGFP control were mock treated (−) or treated (+) with 500 μM H2O2 and lysates were probed as above. Anti-GFP antibodies were used to examine levels of the transfected proteins. For (B-E), a representative experiment from at least 3 independent experiments is shown.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
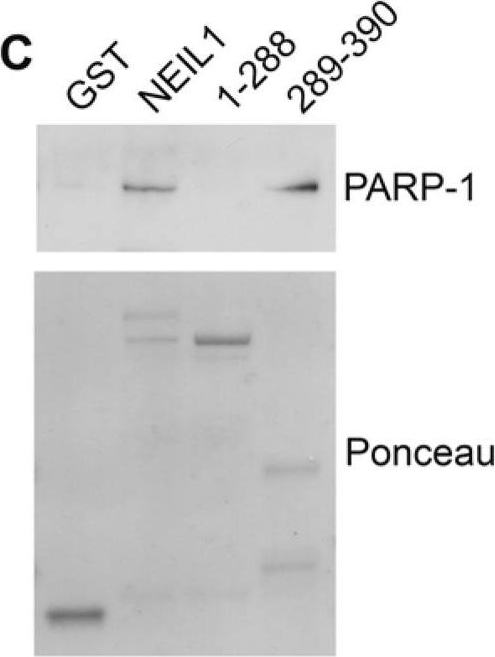
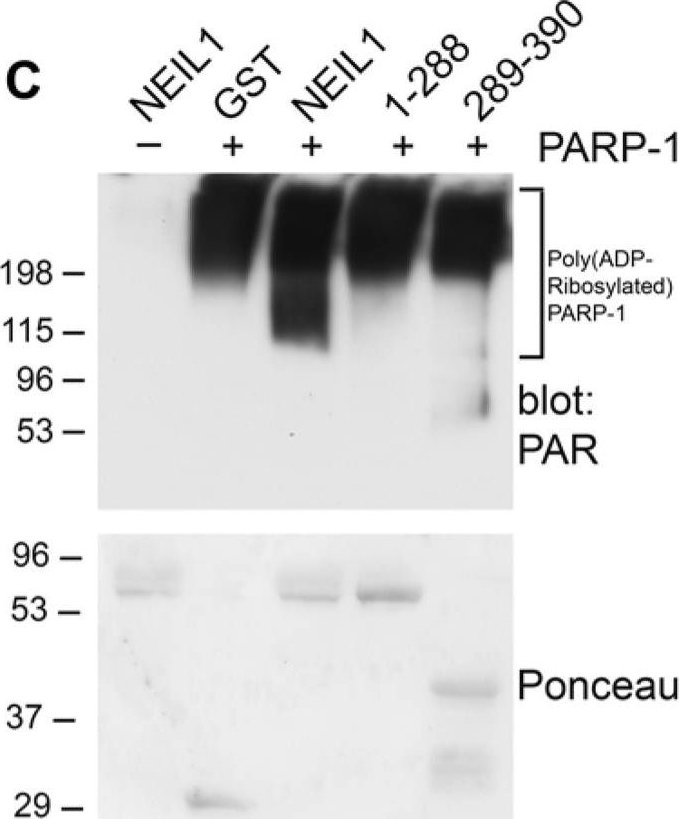
Mapping the interacting regions of PARP-1 and NEIL1(A) Schematic representation of GST-NEIL1 fusion proteins. (B) Purified GST-NEIL1 fusion proteins were analyzed by SDS-PAGE followed by staining with Coomassie Colloidal Blue. (C) PARP-1 binds to the C-terminal 289–390 aa of NEIL1. PARP-1 (250 ng) was incubated with either GST, GST tagged full-length NEIL1 or indicated NEIL1 fragments (1 μg) in an in-vitro binding assay. GST precipitations were probed with anti-PARP-1 antibodies and stained with Ponceau S. ~5% of purified PARP-1 bound to both GST-NEIL and NEIL 289–390aa. (D) NEIL1 binds to the BRCT domain of PARP-1. The DNA binding (41 kDa), BRCT (15 kDa) and catalytic (39 kDa) domains of PARP-1 (1 μg) were incubated with 1 μg GST-NEIL1 (69 kDa) either in the presence (+) or absence (−) of ethidium bromide. GST-NEIL1 precipitations were immunoblotted with anti-His (for the DNA binding and catalytic domains) and anti-BRCT (for BRCT domain) antibodies and then stained with Coomassie to reveal the amount of GST-NEIL1 in the precipitations. The different PARP-1 domains and GST-NEIL1 are indicated by arrows. ~4% of purified BRCT domain bound to GST-NEIL1. (E) A schematic of the different PARP-1 proteins is shown.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
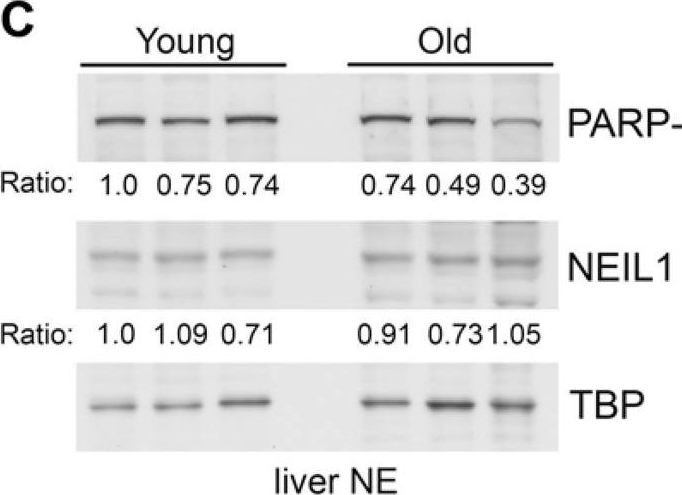
Decreased binding of PARP-1 to GST-NEIL1 with mouse age(A) Liver nuclear extracts were made from 3 different young (7 month) and 3 different old (17 month) mice and used for GST-precipitations with the indicated fusion proteins. Precipitations were probed with anti-PARP-1 and anti-PAR antibodies and stained with Ponceau S to visualize the fusion proteins. The relative amount of PARP-1 binding and PAR levels in precipitations were quantified from immunoblots and normalized to the amount of GST-fusion protein. The numbers below the blots represent the relative level of PARP-1 binding and the histograms in (B) represent the average PARP-1 binding and PAR levels in precipitations from the 3 young and 3 old mice. *P<0.05 comparing young and old mice using Student's t-test. Similar results were obtained in another independent experiment. (C) Liver nuclear extracts from young and old mice were probed by immunoblotting with anti-PARP-1 and anti-NEIL1 antibodies and reprobed with anti-TBP antibodies as a protein loading control. The numbers below the blots represent the level of PARP-1 or NEIL1 normalized to TBP. The histogram in (D) represents the average levels of NEIL1 and PARP-1 + SEM from the 3 young and 3 old mice.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
NEIL1 stimulates the poly(ADP-ribosyl)ation activity of PARP-1(A) PARP-1 activity was measured by determining the amount of PAR deposited on immobilized histones in an ELISA assay. Addition of NEIL1 (33 ng) increased the amount of PAR synthesis by PARP-1 (2 ng). The histogram represents the mean + SEM from three independent experiments. *p < 0.05 compared to control by Student's t-test. (B,C) GST, GST-NEIL1, GST-NEIL1 1–288 aa or GST-NEIL1 289–390 aa (5 μg) were incubated with (+) or without (−) 7.5 ng PARP-1. The immunoblot was stained with Ponceau S and probed with anti-PAR antibodies. (D) WT or NEIL1−/− MEFs were mock treated (−) or treated (+) with 500 μM H2O2. Lysates were probed with anti-PAR antibodies and reprobed with anti-PARP-1 and anti-GAPDH antibodies as a protein loading control. (E) NEIL1−/− MEFs transfected with pEGFP-NEIL1 or pEGFP control were mock treated (−) or treated (+) with 500 μM H2O2 and lysates were probed as above. Anti-GFP antibodies were used to examine levels of the transfected proteins. For (B-E), a representative experiment from at least 3 independent experiments is shown.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
PARP-1 inhibits NEIL1 incision activity(A) NEIL1 (38 nM; 33 ng) was incubated with buffer, NAD+ with or without PARP-1 (180 nM; 400 ng), or with PAR and reacted with a 5′- 32P-labeled oligonucleotide duplex containing a 5OHU lesion for 15 min at 37°C. The cleavage products were analyzed on a 20% denaturing gel containing 7 M urea. Percent incision was calculated by normalizing the amount of cleaved substrate (bottom band) to the amount of uncleaved product (top band). Data was then normalized to the amount of incision activity of NEIL1 alone (100%). (B) Incision assays were performed as in (A) in the presence of the indicated concentrations of PARP-1 with (activated PARP-1) or without NAD+ (PARP-1) and quantified. (C) NEIL1 (33 ng) incision assays were performed in the presence of 400 ng of the indicated PARP-1 domains or full-length PARP-1 in the presence of NAD+ (10 μM). (D) PARP-1 inhibits NEIL1 activity at both 5 nM and 20 nM concentrations of DNA substrate. The histograms in (A) and (B) represent the mean + SEM from three, (C) from five, and (D) from four independent experiments.**p≤0.01 and ***p≤0.001 compared to the incision activity of NEIL1 alone using one-way ANOVA and Tukey’s post-hoc test. (C) NEIL1 was incubated with buffer or PARP-1 with or without NAD+ and incubated with a non-radiolabeled oligonucleotide containing the 5OHU lesion as above. Samples were separated by SDS-PAGE and probed with anti-PAR, anti-PARP-1 and anti-NEIL1 antibodies.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)
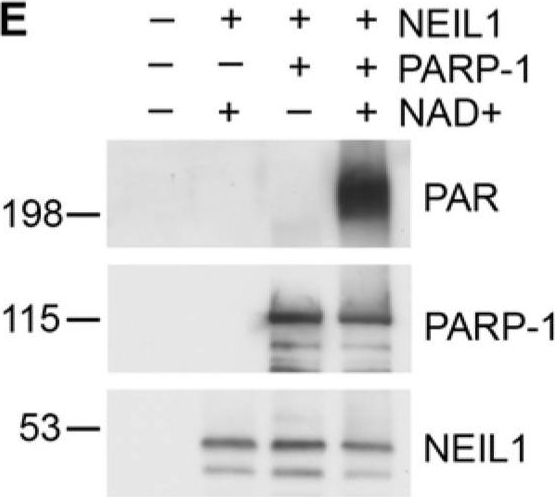
PARP-1 inhibits NEIL1 incision activity(A) NEIL1 (38 nM; 33 ng) was incubated with buffer, NAD+ with or without PARP-1 (180 nM; 400 ng), or with PAR and reacted with a 5′- 32P-labeled oligonucleotide duplex containing a 5OHU lesion for 15 min at 37°C. The cleavage products were analyzed on a 20% denaturing gel containing 7 M urea. Percent incision was calculated by normalizing the amount of cleaved substrate (bottom band) to the amount of uncleaved product (top band). Data was then normalized to the amount of incision activity of NEIL1 alone (100%). (B) Incision assays were performed as in (A) in the presence of the indicated concentrations of PARP-1 with (activated PARP-1) or without NAD+ (PARP-1) and quantified. (C) NEIL1 (33 ng) incision assays were performed in the presence of 400 ng of the indicated PARP-1 domains or full-length PARP-1 in the presence of NAD+ (10 μM). (D) PARP-1 inhibits NEIL1 activity at both 5 nM and 20 nM concentrations of DNA substrate. The histograms in (A) and (B) represent the mean + SEM from three, (C) from five, and (D) from four independent experiments.**p≤0.01 and ***p≤0.001 compared to the incision activity of NEIL1 alone using one-way ANOVA and Tukey’s post-hoc test. (C) NEIL1 was incubated with buffer or PARP-1 with or without NAD+ and incubated with a non-radiolabeled oligonucleotide containing the 5OHU lesion as above. Samples were separated by SDS-PAGE and probed with anti-PAR, anti-PARP-1 and anti-NEIL1 antibodies.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) (2012)










Product Details
| Alternative Name |
Poly(ADP-ribose) polymerase-1 |
|---|---|
| Application |
ELISA, ICC, WB |
| Application Notes |
Detects bands ~116kDa (intact PARP) and ~85kDa (apoptosis-induced cleavage fragment) by Western blot. |
| Clone |
C-2-10 |
| Crossreactivity |
Does not cross-react with chicken PARP. |
| Formulation |
Liquid. In PBS containing 0.02% sodium azide. |
| Host |
Mouse |
| Immunogen |
Purified calf thymus poly(ADP-ribose) polymerase (PARP). |
| Isotype |
IgG1 |
| Purity Detail |
Affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1µg/ml, alkaline phosphatase)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from ascites. |
| Species Reactivity |
Bovine, Hamster, Human, Monkey, Mouse, Rat |
| Specificity |
Recognizes an epitope in the C-terminal part of the DNA binding domain of PARP. |
| UniProt ID |
P18493 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. After opening, prepare aliquots and store at -20°C. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Dry Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- PARP2 promotes Break Induced Replication-mediated telomere fragility in response to replication stress.: Muoio, D., Laspata, N., et al.; Nat. Commun. 15, 2857 (2024), Application(s): WB, Abstract
- Expression of BRCA1, BRCA2, RAD51, and other DSB repair factors is regulated by CRL4WDR70: Z. Mirman, et al.; DNA Repair (Amst.) 113, 103320 (2022), Abstract
- Redox proteome analysis of auranofin exposed ovarian cancer cells (A2780).: Chiappetta, G., Gamberi, T., et al.; Redox Biol. 52, 102294 (2022), Application(s): WB / Reactant(s): Human, Abstract
- Neotelomere formation by human telomerase: Kinzig, C. G., Zakusilo, G., et al.; bioRxiv , (2022), Reactant(s): Human
- New Insights into the Significance of PARP-1 Activation: Flow Cytometric Detection of Poly(ADP-Ribose) as a Marker of Bovine Intramammary Infection: G.D. Matteis, et al.; Cells 10, 599 (2021), Abstract
- Triarylpyridine Compounds and Chloroquine Act in Concert to Trigger Lysosomal Membrane Permeabilization and Cell Death in Cancer Cells.: Martins, I., Müller, A., et al.; Cancers (Basel) 12, (2020), Reactant(s): Human, Abstract
- Combinatory Treatment of Canavanine and Arginine Deprivation Efficiently Targets Human Glioblastoma Cells via Pleiotropic Mechanisms: O. Karatsai, et al.; Cells 9, 2217 (2020), Abstract
- Low dose ionizing radiation strongly stimulates insertional mutagenesis in a γH2AX dependent manner: Zelensky, A. N., Schoonakker, M., et al.; PLoS Genet. 16, e1008550 (2020), Abstract
- Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis.: Fouquerel, E., Barnes, R. P., et al.; Mol. Cell 75, 117 (2019), Application(s): WB, Abstract
- Modulation of the ATM/autophagy pathway by a G-quadruplex ligand tips the balance between senescence and apoptosis in cancer cells: Beauvarlet, J., Bensadoun, P., et al.; Nucleic Acids Res. 47, 2739 (2019), Abstract
- The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination: van den Tempel, N., Laffeber, C., et al.; Oncotarget 8, 44593 (2017), Abstract
- Identification and validation of the dopamine agonist bromocriptine as a novel therapy for high-risk myelodysplastic syndromes and secondary acute myeloid leukemia: Liberante, F. G., Pouryahya, T., et al.; Oncotarget 7, 6609 (2016), Abstract
- Novel p53 target genes secreted by the liver are involved in non-cell-autonomous regulation: Charni, M., Molchadsky, A., et al.; Cell Death Differ. 23, 509 (2016), Abstract
- Autophagy requires poly(adp-ribosyl)ation-dependent AMPK nuclear export: J.M. Rodríguez-Vargas, et al.; Cell Death Differ. 23, 2007 (2016), Application(s): Western blot, MCF7 GFP-LC3 cells, Abstract
- Phosphorylations of Serines 21/9 in Glycogen Synthase Kinase 3α/β Are Not Required for Cell Lineage Commitment or WNT Signaling in the Normal Mouse Intestine.: Forrest, S., Giblett, S., et al.; PLoS One 11, e0156877 (2016), Application(s): WB / Reactant(s): Mouse, Abstract
- PARP inhibitor ABT-888 affects response of MDA-MB-231 cells to doxorubicin treatment, targeting Snail expression: Mariano, G., Ricciardi, M. R., et al.; Oncotarget 6, 15008 (2015), Abstract
- PRIMA-1(MET) induces death in soft-tissue sarcomas cell independent of p53: T. Grellety, et al.; BMC Cancer 15, 684 (2015), Application(s): Immunoblot analysis, Abstract
- Poly(ADP-ribosyl)ation is involved in the epigenetic control of TET1 gene transcription: Ciccarone, F., Valentini, E., et al.; Oncotarget 5, 10356 (2014), Abstract
- Alzheimer’s disease-associated polymorphisms in human OGG1 alter catalytic activity and sensitize cells to DNA damage: K.D. Jacob, et al.; Free Radic. Biol. Med. 63, 115 (2013), Application(s): Western blot, Abstract
- Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging.: Evans, M. K., Barnes, J., et al.; Aging (Albany NY) 4, 674 (2012), Application(s): WB, Abstract
- The Akt inhibitor triciribine sensitizes prostate carcinoma cells to TRAIL-induced apoptosis: Dieterle, A., Orth, R., et al.; Int. J. Cancer 125, 932 (2009), Abstract
- Regulation of poly(ADP-ribose) polymerase-1 functions by leukocyte elastase inhibitor/LEI-derived DNase II during caspase-independent apoptosis: C. Leprête, et al.; Int. J. Biochem. Cell Biol. 41, 1046 (2009), Abstract
- Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment: Geserick, P., Hupe, M., et al.; J. Cell Biol. 187, 1037 (2009), Abstract
- CCCTC-binding Factor Activates PARP-1 Affecting DNA Methylation Machinery: T. Guastafierro, et al.; J. Biol. Chem. 283, 21873 (2008), Abstract — Full Text
- Nuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunction: G. Cipriani, et al.; J. Biol. Chem. 280, 17227 (2005), Abstract — Full Text
- Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio: D. Javelaud & F. Besançon; J. Biol. Chem. 277, 37949 (2002), Abstract — Full Text
- Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases: S. Gobeil, et al.; Cell Death Differ. 8, 588 (2001), Abstract
- Selective loss of poly(ADP-ribose) and the 85-kDa fragment of poly(ADP- ribose) polymerase in nucleoli during alkylation-induced apoptosis of HeLa cells: R. Alvarez-Gonzalez, et al.; J. Biol. Chem. 274, 32122 (1999), Abstract — Full Text
- Characterization of anti-peptide antibodies directed towards the automodification domain and apoptotic fragment of poly (ADP-ribose) polymerase: P.J. Duriez, et al.; Biochim. Biophys. Acta 1334, 65 (1997), Abstract
- Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis: R. Datta, et al.; J. Biol. Chem. 272, 1965 (1997), Abstract
- FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis: A.M. Chinnaiyan, et al.; J. Biol. Chem. 271, 4961 (1996), Abstract — Full Text
- Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells: G.M. Shah, et al.; Biochem. Biophys. Res. Commun. 229, 838 (1996), Abstract
- Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution: Y.A. Lazebnik, et al.; PNAS 92, 9042 (1995), Abstract — Full Text
- Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE: Y.A. Lazebnik, et al.; Nature 371, 346 (1994), Abstract
- Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis: S.H. Kaufmann, et al.; Cancer Res. 53, 3976 (1993), Abstract
- Structural and functional analysis of poly(ADP ribose) polymerase: an immunological study: D. Lamarre, et al.; Biochim. Biophys. Acta 950, 147 (1988), Abstract
Related Products

| Alternative Name | Poly(ADP-ribose) polymerase-1 |
|---|---|
| Application | ELISA, IHC, WB |
| Host | Mouse |
| Isotype | IgG1 |
| Species Reactivity | Bovine, Hamster, Human, Monkey, Mouse, Rat |

| Alternative Name | Poly(ADP-ribose) polymerase, PARP-1 |
|---|---|
| Application | ELISA, WB |
| Host | Rabbit |
| Species Reactivity | Bovine, Human, Mouse, Rat |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?