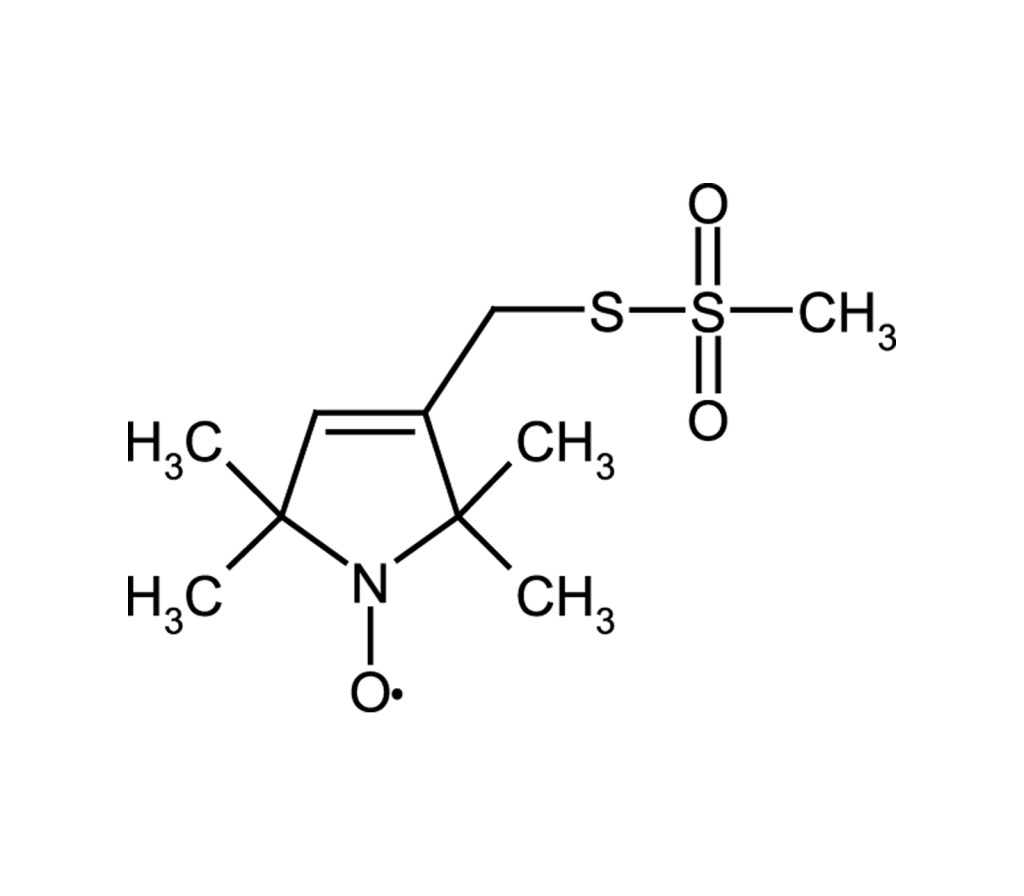
Thiol-specific spin label
Highly reactive thiol-specific spin label. Has been used to label cysteine residues in proteins (site-directed labeling, SDS-labeling). Allows protein structure and protein dynamics determination as well as the study of protein-protein and protein-oligonucleotide interactions.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
Product Details
| Alternative Name |
(1-Oxyl-2,2,5,5-tetramethylpyrroline-3-methyl) methanethiosulfonate |
|---|---|
| Appearance |
Yellow crystalline solid. |
| CAS |
81213-52-7 |
| Formula |
C10H18NO3S2 |
| MW |
264.3 |
| Purity |
≥98% (HPLC) |
| Solubility |
Soluble in water, methanol, 100% ethanol, DMSO, acetonitrile or acetone. |
Handling & Storage
| Use/Stability |
As indicated on product label or CoA when stored as recommended. |
|---|---|
| Handling |
Protect from light. |
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Cholesterol twists the transmembrane Di-Gly region of amyloid-precursor protein: D.T. Wang, et al.; PNAS Nexus 2, pgad162 (2023), Abstract — Full Text
- Structural Insights into the Binding and Degradation Mechanisms of Protoporphyrin IX by the Translocator Protein TSPO: P.S. Yeh, et al.; JACS Au 3, 2918 (2023), Abstract
- Rapid Scan Electron Paramagnetic Resonance Spectroscopy Is a Suitable Tool to Study Intermolecular Interactions of Intrinsically Disordered Protein: J. Dröden, et al.; Biology 12, 79 (2023), Abstract
- Structure and mechanism of human cystine exporter cystinosin: X. Guo, et al.; Cell 185, 3739 (2022), Abstract
- Nanodisc Lipids Exhibit Singular Behaviors Implying Critical Phenomena: P.S. Ho, et al.; Langmuir 49, 15372 (2022), Abstract
- Proton-driven alternating access in a spinster lipid transporter: R. Dastvan, et al.; Nat. Commun. 13, 5161 (2022), Abstract
- Location of the cross-β structure in prion fibrils: A search by seeding and electron spin resonance spectroscopy: B.K.Y. Chu, et al.; Protein Sci. 31, e4326 (2022), Abstract
- Protein and solutes freeze-concentration in water/glycerol mixtures revealed by pulse EPR: N. Isaev, et al.; Eur. J. Pharm. Biopharm. 169, 44 (2021), Abstract
- A molecular switch regulating transcriptional repression and activation of PPARγ: Shang, J., Mosure, S. A., et al.; Nat. Commun. 11, 956 (2020), Abstract
- Conformational transitions of the sodium-dependent sugar transporter, vSGLT: A. Paz, et al.; PNAS 115, E2742 (2018), Abstract — Full Text
- Lipids modulate the conformational dynamics of a secondary multidrug transporter: C. Martens, et al.; Nat. Struct. Mol. Biol. 23, 744 (2016), Application(s): Spin-labeling, after LmrP mutant purification, Abstract
- A new structural model of Alzheimer’s Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling: L. Gu, et al.; J. Struct. Biol. 194, 61 (2016), Application(s): Cell culture, Abstract
- Protonation-dependent conformational dynamics of the multidrug transporter EmrE: R. Dastvan, et al.; PNAS 113, 1220 (2016), Abstract — Full Text
- Conformational dynamics of the nucleotide binding domains and the power stroke of a heterodimeric ABC transporter: S. Mishra et al.; eLife 3, e02740 (2014), Application(s): Size-exclusion chromatography, Abstract — Full Text
- The Structure of the RLIP76 RhoGAP-Ral Binding Domain Dyad: Fixed Position of the Domains Leads to Dual Engagement of Small G Proteins at the Membrane: K.V. Rajasekar, et al.; Structure 21, 2131 (2013), Application(s): Mass spectrometry, Assay, Abstract — Full Text
- Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils: L. Gu, et al.; J. Neurochem. 126, 305 (2013), Application(s): MALDI-TOF mass spectrometry, Abstract — Full Text
- Hierarchical Organization in the Amyloid Core of Yeast Prion Protein Ure2: S. Ngo, et al.; J. Biol. Chem. 286, 29691 (2011), Application(s): MALDI-TOF mass spectrometry, Abstract — Full Text
- Calcium structural transition of human cardiac troponin C in reconstituted muscle fibres as studied by site-directed spin labelling: M. Nakamura, et al.; J. Mol. Biol. 348, 127 (2005), Abstract
- Inter- and intra-molecular distances determined by EPR spectroscopy and site-directed spin labeling reveal protein-protein and protein-oligonucleotide interaction: H.J. Steinhoff; Biol. Chem. 385, 913 (2004), Abstract
- Spontaneous refolding of the pore-forming Colicin A toxin upon membrane association as studied by X-band and W-band high-field electron paramagnetic resonance spectroscopy: A. Savitski, et al.; J. Phys. Chem. B 108, 9541 (2004)
- Methods for study of protein dynamics and protein-protein interaction in protein-ubiquitination by electron paramagnetic resonance spectroscopy: H.J. Steinhoff; Front. Biosci. 7, c97 (2002), Abstract
- Protein structure determination using long-distance constraints from double-quantum coherence ESR: study of T4 lysozyme: P.P. Borbat, et al.; JACS 124, 5304 (2002), Abstract
- Pressure-induced thermostabilization of glutamate dehydrogenase from the hyperthermophile Pyrococcus furiosus: M.M. Sun, et al.; Protein Sci. 8, 1056 (1999), Abstract
- A novel reversible thiol-specific spin label: papain active site labeling and inhibition: L.J. Berliner, et al.; Anal. Biochem. 119, 450 (1982), Abstract
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?