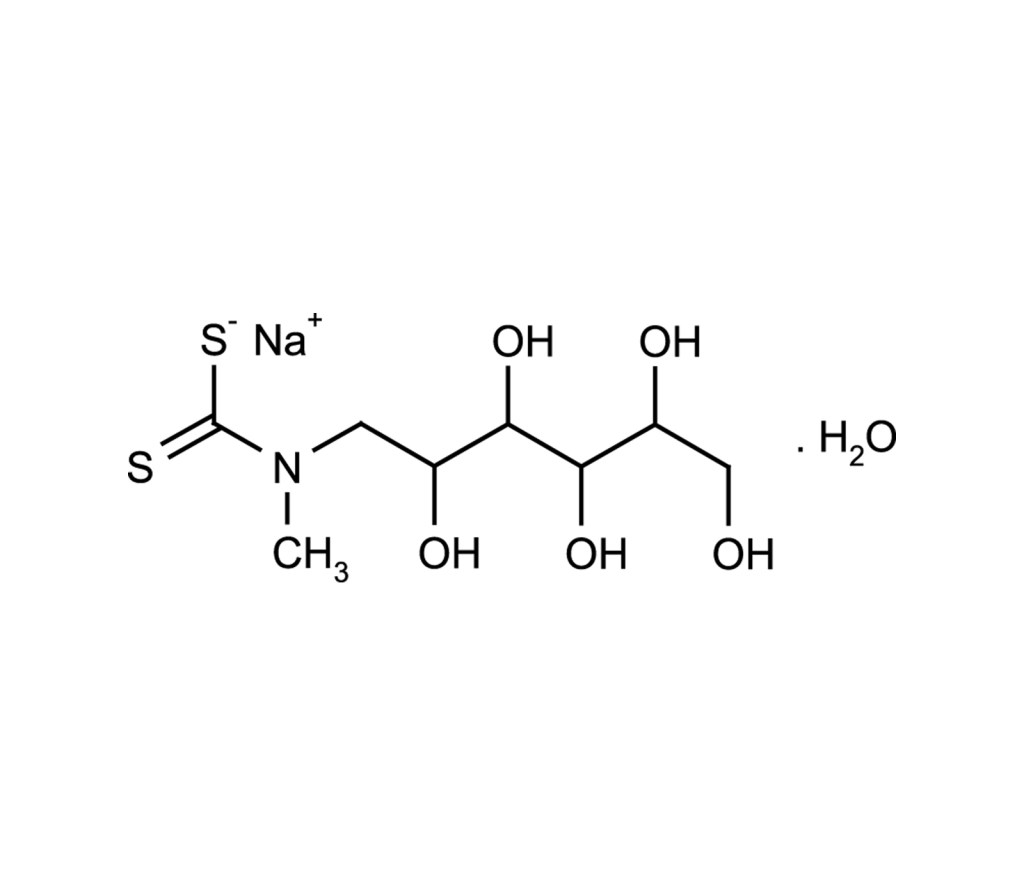
Together with FeSO4 MGD is a useful component for the formation of the MGD2-Fe2+complex, which is an excellent nitric oxide (NO) spin-trapping reagent. The MGD2-Fe2+complex is quite unstable, especially in the presence of dissolved oxygen. Thus, the complex should be used immediately after being made. An excess (usually 5-fold excess), of MGD to Fe2+ is used for making the complex with FeSO4 to give a more stable complex solution. Acidic conditions should be avoided because dithiocarbamate tends to decompose forming toxic carbon disulfide. It was reported that MGD and Fe(MGD)2 do not exhibit toxicity up to 8mmol/kg and 0.3mmol/kg, respectively.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?