Manuals And Inserts
Specific Protocol
Assay of LSD1 (Catalog # BML-SE544) by a Peroxidase-Coupled Assay with Histone H3 Dimethyl Lysine-4 Peptide (H3 K4Me2), Cat. # BML-P256)
Components of the Assay:
LSD1 Assay Buffer (LAB): (25mM KH2PO4KOH, pH7.8, 5% v/v glycerol)
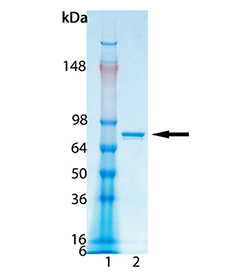
LSD1 (Cat. # BML-SE544): Dilute to enough LSD1 to 0.05μg/μl with LSD1 Assay Buffer to provide 10μl per well. Keep on ice until use.
4-Aminoantipyrine (4-AA): Prepare a 5mM stock in dH2O. Store at -20°C or short term at 4°C.
3,5-dichloro-2-hydroxybenzenesulfonic acid (DCHBS): Prepare a 50mM stock in dH2O. Store at -20°C or short term at 4°C.
Horse Radish Peroxidase (HRP): Prepare a 100μM stock in dH2O and keep on ice. Prepare fresh daily and discard remainder.
Peroxidase Reaction Mix (PRM): The peroxide generated by the LSD1-catalyzed demethylation of H3 K4Me2 will produce, in an HRP-catalyzed reaction, a colored adduct of 4-AA and DCHBS with an extinction coefficient of 26,000 M-1cm-1 at 515nm. PRM is: 7.5μM HRP, 0.25mM 4-AA, 2.5mM DCHBS diluted in LAB. Plan to prepare enough PRM to provide 40μl per well. The 0.05μg/μl LSD1 dilution (10μl) will be added to the 40μl of PRM and later 50μl of a 2x substrate solution will be added (see below). If, for example, you plan to pre-incubate the LSD1 with a potential inhibitor prior to addition of the peptide substrate, it may be desirable to prepare aliquots of PRM with the inhibitor added to 2.5 times its final concentration.
Histone H3 Dimethyl Lysine-4 Peptide (H3 K4Me2) (Cat. # BML-P256): Prepare a 0.5mM stock of of H3 K4Me2 by dissolving 0.5mg net peptide (1 vial of Cat. # BML-P256) in 438μl of LSD1 Assay Buffer.
2x Substrate Solution: Prepare a 2x substrate solution by diluting the 0.5 mM H3 K4Me2. Each assay well will require 50 μl (see below). For example, prepare 1 ml of 20 μM substrate (for final 10 μM) by mixing 40μl of 0.5 mM H3 K4Me2 and 960μl LAB. (NOTE: LSD1’s Km for H3 K4Me2 is 9.2μM. Therefore, a convenient substrate concentration for inhibitor screening Might be 10μM H3 K4Me2, while 100μM would be more suitable for a specific activity measurement at a saturating substrate concentration.) Warm to 30°C before use. If, for example, you plan to expose LSD1 to a potential inhibitor simultaneously with the addition of the peptide substrate, it may be desirable to prepare aliquots of 2x Substrate Solution with the inhibitor added to 2.0 times its final concentration.
Clear-well ½-volume 96-well microplate
Reaction Condition Examples:
1) Designate wells for two reactions: one well for +LSD1 and one no-enzyme control.
2) Add 40μl of PRM to both wells. Allow the microplate to equilibrate to assay temperature (30°C).
3) Add 10μl of diluted LSD1 (BML-SE544, 0.05μg/μl) to the +LSD1 well and 10μl LAB to the no-enzyme well.
4) To start reactions, add 50μl of the 2x Substrate (30°C) to both wells.
5) Data analysis: Determine the rate of the enzyme reaction in mOD/min at 515nm, by, for example, determining the slope of the initial, linear part of a graph of absorbance (mOD units) versus time (min.) The extinction coefficient of the colored reaction product is 26,000 M-1cm-1 and the optical path-length of 100μl in a ½-volume 96-well microplate is ~0.5 cm. (NOTE: Some plate-reading spectrophotometers have a feature that allows the path-length to measured empirically for each well.) If a path-length of 0.50cm is assumed, rates in mOD515nm/min may converted to pmol/min with a conversion factor of 7.7pmol/mOD515nm.
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?