LRRC32 (leucine rich repeat containing 32; also known as GARP or Garpin; Glycoprotein A repetitions predominant) is a glycoprotein expressed on the cell surface of megakaryocytes, platelets and activated regulatory T (Treg) cells.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
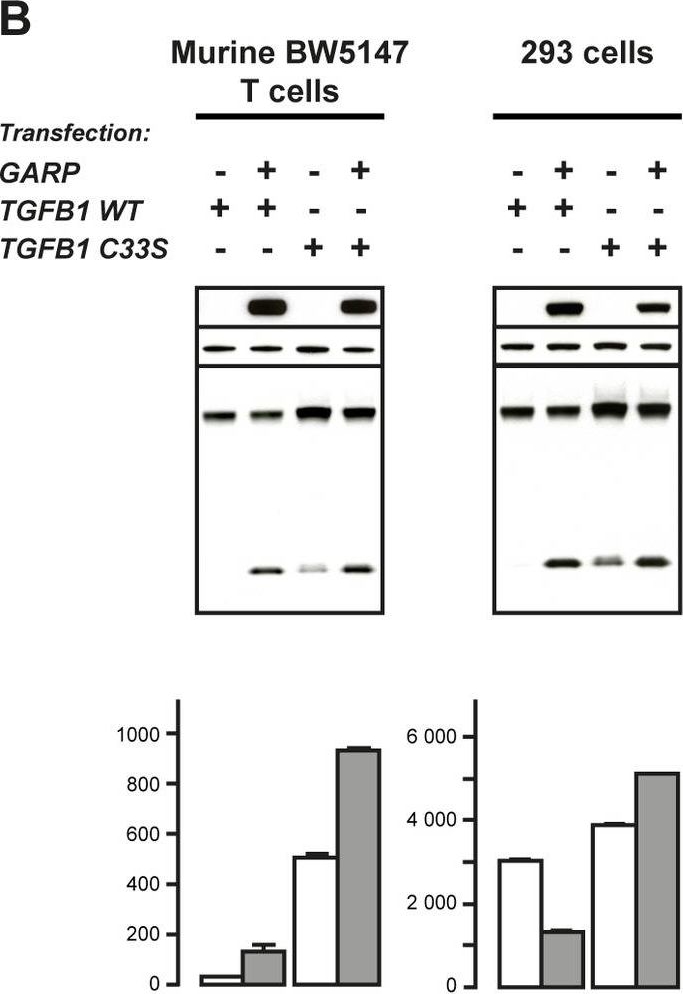
GARP increases cleavage of the pro-TGF-β1 precursor and secretion of latent TGF-β1 in T lymphocytes.Cell lysates were analyzed by WB after SDS-PAGE under reducing conditions with antibodies against GARP, β-actin and a C-terminal epitope of the TGF-β1 peptide (top panels). Supernatants were treated or not with acid and analyzed by ELISA to measure concentrations of total (latent + active) and active TGF-β1, respectively (bottom panels). Total TGF-β1 detected in the acid-treated samples corresponds to latent TGF-β1 because no active TGF-β1 was detected in the non-treated samples. Values represent means of duplicates + SD. A. Analysis of human T cell lines transduced or not with lentiviruses coding GARP or GFP. T cells were left resting (Rest) or stimulated for 24 hours with anti-CD3/CD28 antibodies (Stim) in serum-free medium. B. Analysis of stable clones of murine BW5147 T cells and 293 cells transiently transfected with GARP and WT or C33S mutant TGFB1. Untransfected BW5147 and 293 cells express low levels of endogenous TGF-β1 that are not detectable by WB in these conditions (not shown). By comparison to WT, transfection of mutant C33S results in increased production of total TGF-β1 (pro- + mature), as previously described [49].
Image collected and cropped by CiteAb under a CC-BY license from the following publication: GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One (2013)
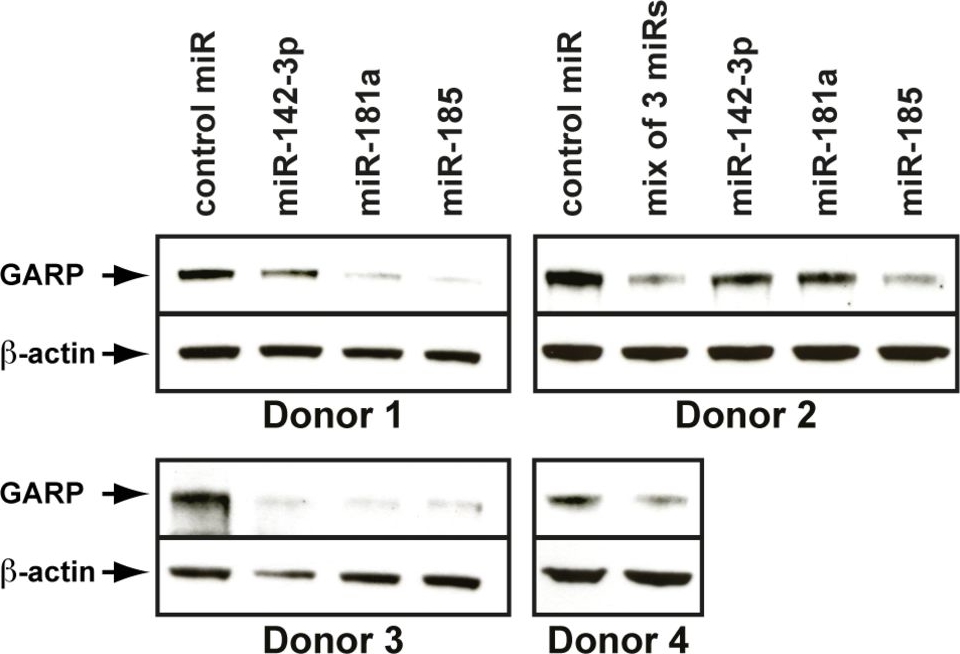
Endogenous GARP levels in Tregs are reduced after transfection of miR-181a, miR-142-3p and miR-185 mimics.Polyclonal CD4+CD25+CD127lo populations were purified from human PBMCs and amplified invitro. Amplified cells were electroporated with the indicated miRNA mimics and stimulated 6 hours later with anti-CD3/CD28 antibodies. Cell lysates were collected 24 hours later and analyzed by WB with anti-GARP and anti-β-ACTIN antibodies.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One (2013)
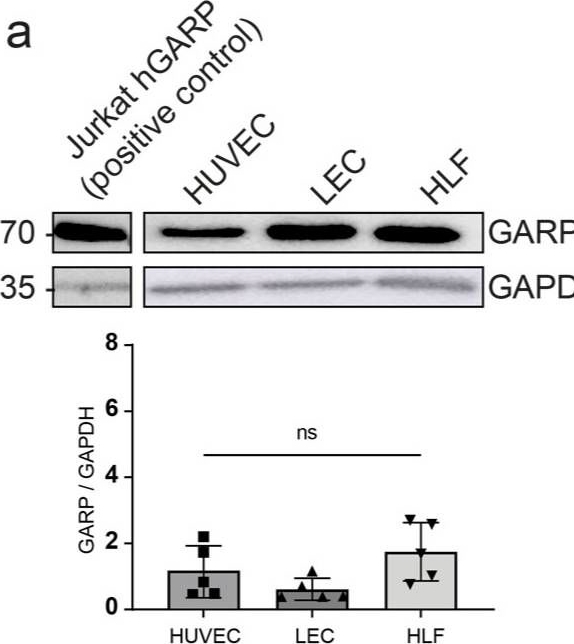
Evaluation of GARP in HUVEC, LEC, and HLF cells cultured under basal conditions. (a) Western blot analysis of GARP expression. The blot is a representative blot out of 4 independent experiments. The bar graph shows the quantification of GARP protein levels relative to the GAPDH protein signal (GARP/GAPDH signals) (n = 4, means ± SD, n.s. determined by one-way ANOVA). (b) Flow cytometry analysis of GARP at the surface of primary cells. Jurkat cells overexpressing GARP (Jurkat−hGARP) were used as a positive control. The isotype control is represented in grey, and the positive signal is depicted in red as a percentage of the maximum. The relative MFI of GARP in flow cytometry is represented with a bar graph (n ≥ 3, means ± SD, n.s., no significance, determined by one-way ANOVA).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Spatial Distribution of Non-Immune Cells Expressing Glycoprotein A Repetitions Predominant in Human and Murine Metastatic Lymph Nodes. Cancers (Basel) (2023)
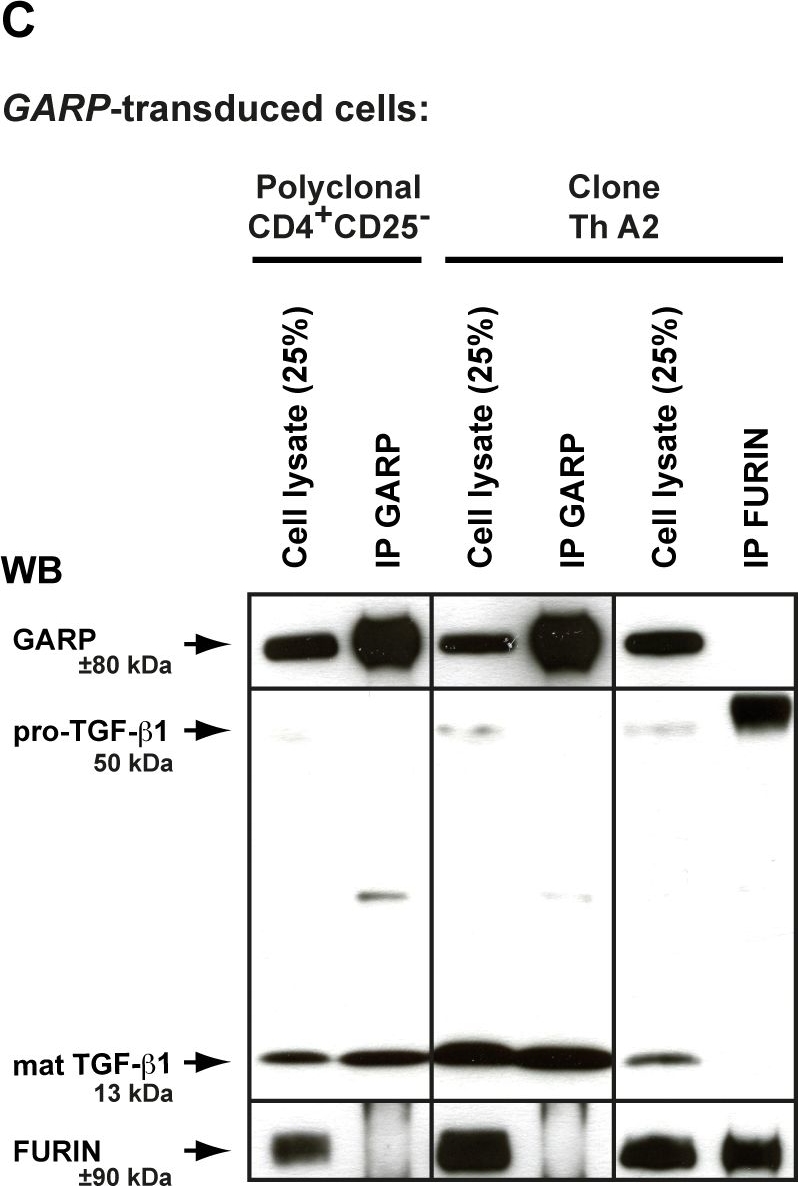
GARP does not increase FURIN expression or activity, and does not co-immunoprecipitate with FURIN.A. Expression of FURIN mRNA and protein were analyzed by RT-qPCR and WB in the human cells described in Figure 2. B. FURIN activity was measured 24 hours after transfection of 293 cells. Lysates of transfected cells were incubated with a FURIN fluorogenic substrate directly (top panel), or after capture on plastic-coated anti-FURIN antibody (bottom panel), to measure FURIN-like or FURIN specific activity, respectively. Graphs show mean fluorescence intensity at the indicated time (min) after addition of the substrate. The FURIN inhibitor Dec-RVKR-CMK was added to one condition to verify the specificity of the assay. C. Lysates of cells described in Figure 2 were immunoprecipitated with anti-GARP (IP GARP) or anti-FURIN (IP FURIN) antibodies. Immunoprecipitation products or total cell lysates (25% of input used for IPs) were analyzed by WB with anti-GARP, anti-TGF-β or anti-FURIN antibodies, as indicated.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One (2013)
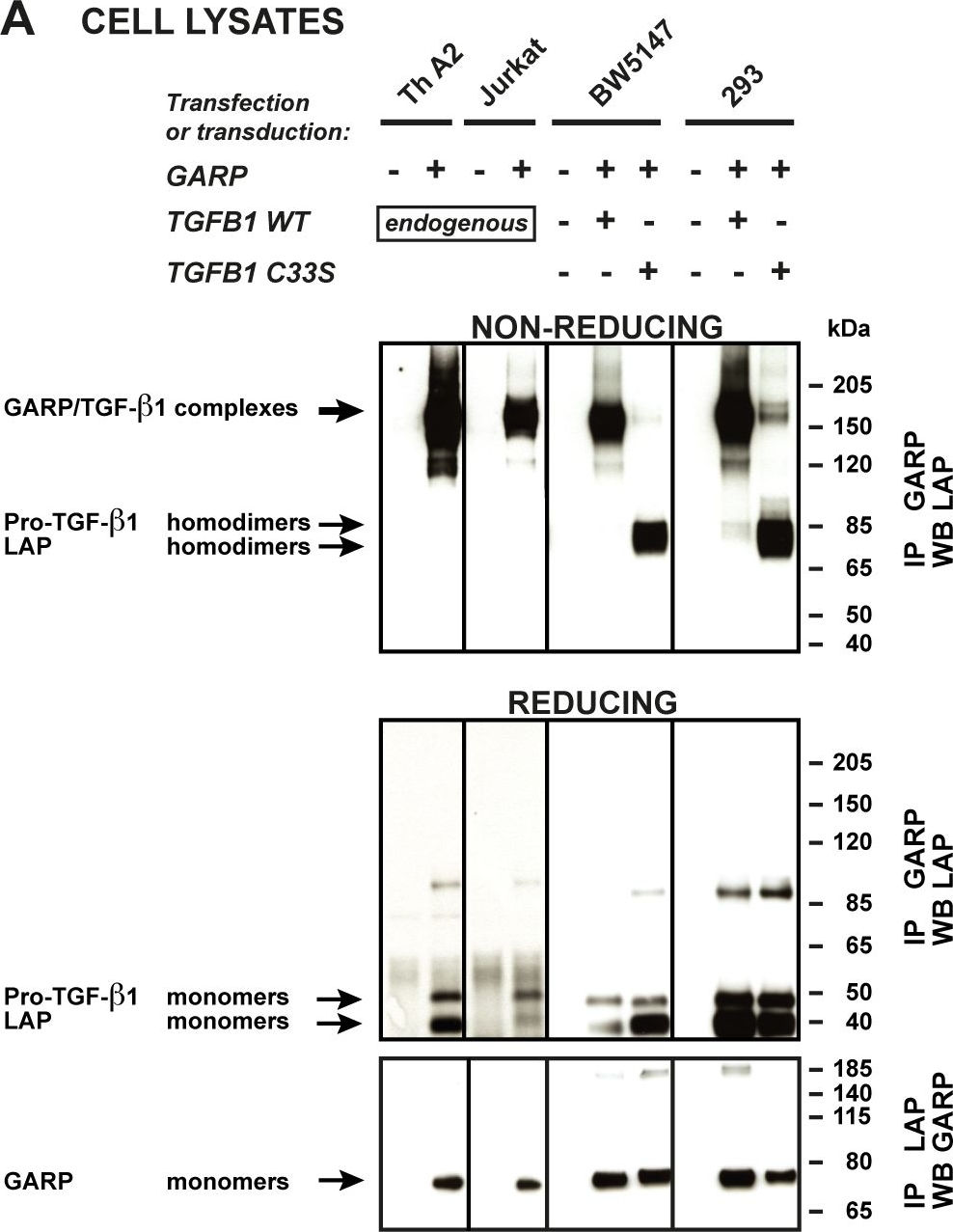
Disulfide-linked GARP/TGF-β1 complexes are released in the supernatant of T cells, but not 293 cells.A. Cells described in Figure 2 were lysed and immunoprecipitated (IP) with anti-GARP or anti-LAP antibodies. IP products were submitted to SDS-PAGE under non-reducing or reducing conditions, followed by WB with anti-LAP antibodies (top and middle panels), or anti-GARP antibodies (bottom panels). Pro-TGF-β1 and LAP homodimers in the top panels are not clearly resolved, but can be distinguished better with longer migrations or higher concentrations of polyacrylamide. The +/- 85-90 kDa bands that appear in the middle panel correspond to non-specific bands, or to incompletely reduced pro-TGF-β1. B. Cells (2×106/ml for murine and human T cells, 2.5×105/ml for transfected 293 cells) were incubated in serum free medium during 24 hours. Different cell concentrations were used to adjust for the different amounts of secreted TGF-β1 (see Figure 2). Human Th A2 and Jurkat cells were stimulated with anti-CD3/CD28 antibodies to increase secretion. Supernatants (0.5-10 µl) were analyzed by WB under non-reducing conditions with anti-GARP and anti-LAP antibodies. * Band that also appears when the secondary anti-IgG2b-HRP antibody is used alone (without anti-GARP antibody), due to cross reactivity against the anti-CD3/CD28 antibodies used for T cell stimulation.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One (2013)
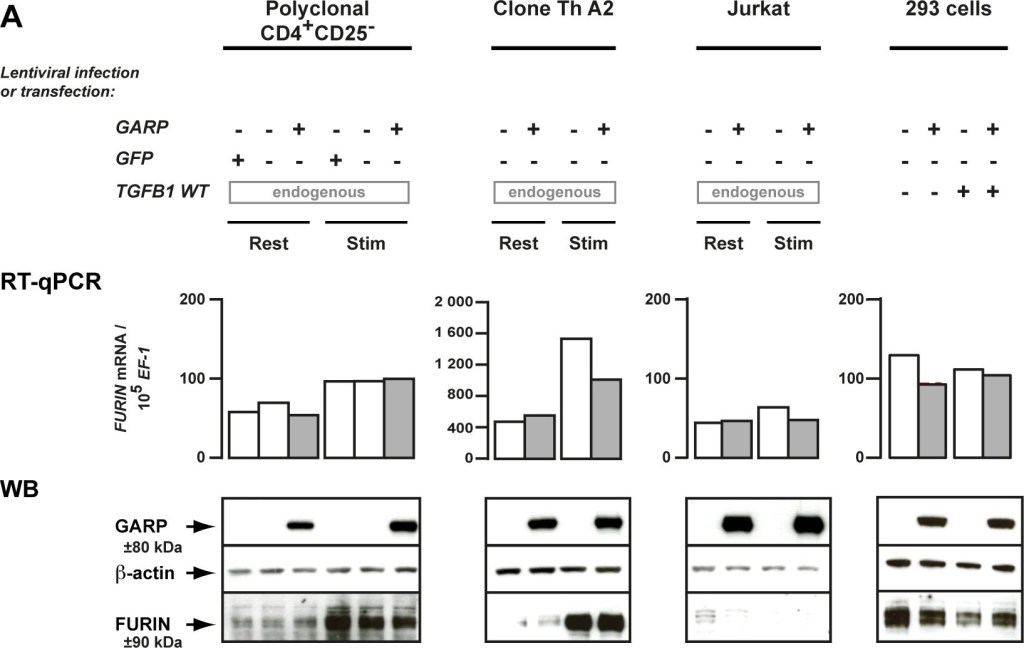
GARP does not increase FURIN expression or activity, and does not co-immunoprecipitate with FURIN.A. Expression of FURIN mRNA and protein were analyzed by RT-qPCR and WB in the human cells described in Figure 2. B. FURIN activity was measured 24 hours after transfection of 293 cells. Lysates of transfected cells were incubated with a FURIN fluorogenic substrate directly (top panel), or after capture on plastic-coated anti-FURIN antibody (bottom panel), to measure FURIN-like or FURIN specific activity, respectively. Graphs show mean fluorescence intensity at the indicated time (min) after addition of the substrate. The FURIN inhibitor Dec-RVKR-CMK was added to one condition to verify the specificity of the assay. C. Lysates of cells described in Figure 2 were immunoprecipitated with anti-GARP (IP GARP) or anti-FURIN (IP FURIN) antibodies. Immunoprecipitation products or total cell lysates (25% of input used for IPs) were analyzed by WB with anti-GARP, anti-TGF-β or anti-FURIN antibodies, as indicated.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One (2013)






Product Details
| Alternative Name |
Leucine-rich repeat-containing protein 32, GARP, Garpin, Glycoprotein A repetitions predominant |
|---|---|
| Application |
ELISA, IP, WB |
| Application Notes |
Cited applications include flow cytometry. |
| Clone |
Plato-1 |
| Formulation |
Liquid. In PBS containing 50% glycerol and 0.02% sodium azide. |
| Host |
Mouse |
| Immunogen |
Recombinant human LRRC32 (aa 20-627). Detects a band of ~100kDa (Fc Fusion ALX-522-117) by Western blot. Native protein is ~72kDa. |
| Isotype |
IgG2b |
| Purity Detail |
Protein G-affinity purified. |
| Recommendation Dilutions/Conditions |
Immunoprecipitation (1:200)Western Blot (1:1,000)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from concentrated hybridoma tissue culture supernatant. |
| Species Reactivity |
Human, Mouse |
| Technical Info / Product Notes |
1 test means: 1µl of MAb is used to stain 500’000 cells in a sample volume of 50µl. |
| UniProt ID |
Q14392 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. |
|---|---|
| Handling |
For long term storage keep unconjugated antibody at -20°C. Avoid freeze/thaw cycles. Protect from light. |
| Short Term Storage |
+4°C |
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Targeted radionuclide therapy against GARP expressing T regulatory cells after tumour priming with external beam radiotherapy in a murine syngeneic model: Bellaye, P. S., Dias, A. M., et al.; Heliyon 10, e39543 (2024), Abstract
- Spatial Distribution of Non-Immune Cells Expressing Glycoprotein A Repetitions Predominant in Human and Murine Metastatic Lymph Nodes.: Rouaud, L., Baudin, L., et al.; Cancers (Basel) 15, (2023), Application(s): WB, Abstract
- Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism: M. Zhang, et al.; Signal Transduct. Target Ther. 6, 366 (2021), Application(s): IHC, Abstract
- Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8: J. Stockis, et al.; PNAS 114, E10161 (2017), Application(s): WB, Abstract
- Cutting Edge: Active TGF-β1 Released from GARP/TGF-β1 Complexes on the Surface of Stimulated Human B Lymphocytes Increases Class-Switch Recombination and Production of IgA: Dedobbeleer, O., Stockis, J., et al.; J. Immunol. 199, 391 (2017), Abstract
- A key role of GARP in the immune suppressive tumor microenvironment: S.A. Hahn, et al.; Oncotarget 7, 42996 (2016), Application(s): Western blot, Abstract — Full Text
- Surface Expression of TGFβ Docking Receptor GARP Promotes Oncogenesis and Immune Tolerance in Breast Cancer: A. Metelli, et al.; Cancer Res. 76, 7106 (2016), Application(s): IHC, Abstract — Full Text
- Lysosomal-associated Transmembrane Protein 4B (LAPTM4B) Decreases Transforming Growth Factor β1 (TGF-β1) Production in Human Regulatory T Cells: C. Huygens, et al.; J. Biol. Chem. 290, 20105 (2015), Abstract — Full Text
- Feline glycoprotein A repetitions predominant anchors transforming growth factor beta on the surface of activated CD4(+)CD25(+) regulatory T cells and mediates AIDS lentivirus-induced T cell immunodeficiency: M.M. Miller, et al.; AIDS Res. Hum. Retroviruses 29, 641 (2013), Application(s): Flow cytometry, Abstract — Full Text
- Interleukin-21 (IL-21) synergizes with IL-2 to enhance T-cell receptor-induced human T-cell proliferation and counteracts IL-2/transforming growth factor-β-induced regulatory T-cell development: A. Battaglia, et al.; Immunology 139, 109 (2013), Application(s): Flow cytometry, Abstract — Full Text
- GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells: E. Gauthy, et al.; PLoS One 8, e76186 (2013), Application(s): WB, WB post IP / Reactant(s) Human, Abstract — Full Text
- CD4(+)CD25(+)Foxp3(+)IFNγ(+) Treg are immunosuppressive in vitro and increase with intensity of the alloresponse in pretransplant MLC: V. Daniel, et al.; Transpl. Immunol. 27, 114 (2012), Application(s): Flow cytometry, Abstract
- In vitro HIV infection impairs the capacity of myeloid dendritic cells to induce regulatory T cells.: Moreno-Fernandez, M. E., Chougnet, C. A., et al.; PLoS One 7, e42802 (2012), Application(s): FC/FACS / Reactant(s): Human, Abstract
- Therapeutic potential of FOXP3+ regulatory T cells and their interactions with dendritic cells: D.Q. Tran & E.M. Shevach; Hum. Immunol. 70, 294 (2009), Abstract
- Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells: R. Wang, et al.; PNAS 106, 13439 (2009), Abstract
- GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells: D.Q. Tran, et al.; PNAS 106, 13445 (2009), Abstract
Related Products
| Alternative Name | Leucine-rich repeat-containing protein 32, GARP, Garpin, Glycoprotein A repetitions predominant |
|---|---|
| Purity | ≥90% (SDS-PAGE) |
| Source | Produced in CHO cells. Human LRRC32 (aa 20-627) is fused to the Fc portion of human IgG1. |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
