The Hsp90 family of heat shock proteins represents one of the most abundantly expressed and highly conserved families of cellular chaperones whose expression can be upregulated under conditions of cellular stress, and includes cytoplasmic (Hsp90-alpha/beta), ER (grp94), and mitochondrial (TRAP1) localized members. Structurally, Hsp90 is characterized by an N-terminal ATP-binding domain, a medial substrate-binding domain, and a C-terminal dimerization motif. Hsp90 dimers function in cooperation with cochaperones (e.g. Hsp40, Hsp70, Hop, p23) to stabilize a multitude of client protein substrates, including steroid hormone receptors, protein kinases, and transcription factors. The essential binding and hydrolysis of ATP by Hsp90 is inhibited by ansamycin drugs (e.g. geldanamycin, 17-AAG) which occupy the N-terminal Hsp90 nucleotide-binding pocket. Many Hsp90 client proteins such as erbB2/Her-2, c-raf, bcr-abl, p53, and hTERT, are members of well characterized oncogenic pathways, making Hsp90 inhibitors useful anticancer agents.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
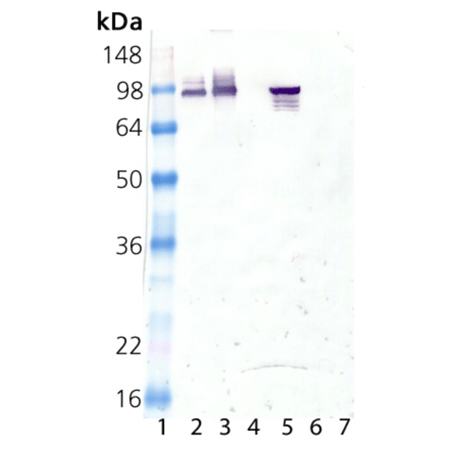
Western blot analysis of HSP90α, mAb (9D2) (Prod. No. ADI-SPA-840):
Lane 1: MW marker, Lane 2: HSP90 native protein (Prod. No. ADI-SPP-770),
Lane 3: HSP90 alpha recombinant protein (Prod. No. ADI-SPP-776),
Lane 4: HSP90 beta recombinant protein (Prod. No. ADI-SPP-777),
Lane 5: HeLa Cell Lysate (heat shocked) (Prod. No. ADI-LYC-HL101),
Lane 6: PC-12 Cell Lysate (heat shocked) (Prod. No. ADI-LYC-PC101),
Lane 7: 3T3 Cell Lysate (heat shocked) (Prod. No. ADI-LYC-3T101).
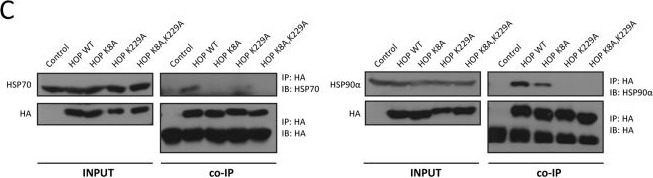
LRET assays of the interactions of TPR mutants of HOP with HSP70 and HSP90. (A) Sequence alignment of the relevant portions of the TPR of CHIP and of TPR1 and TPR2A of HOP; K30 of CHIP, which is known to be important for binding HSP70 and HSP9032,33, is highlighted with a blue arrow. (B) Immunoblot analysis of TPR point mutants; HA-tagged constructs were transiently expressed in HEK293T cells and revealed using both anti-HOP and anti-HA antibodies as indicated with GAPDH as loading control. (C) Co-immunoprecipitation experiments to check the association between HOP mutants and endogenous HSP70 and HSP90; IP, immunoprecipitation; co-IP, coimmunoprecipitation; IB, immunoblot with indicated antibody. The uncropped original images of the immunoblots shown in panels B and C are presented in Supplementary Fig. S2. (D) Luminescence patterns of Tb3+ bound wild-type (LBT-TPR2A WT) and point mutant (LBT-TPR2A K229A). (E) Intrinsic EGFP fluorescence and LRET profiles for wild-type (WT) and point mutant TPR2A. (F) LRET titration experiment comparing the binding of wild-type and mutant TPR2A to HSP90. TPR2A WT and K229A (10 μM) loaded with equimolar Tb3+ were titrated with increasing concentrations of EGFP-C90 (0–6 μM). The Scatchard plot of the normalized LRET from three independent experiments represents means ± SEM. (G) Intrinsic TagRFP fluorescence and LRET profiles for wild-type (WT) and point mutant TPR1. In some panels, the position of the LRET signal is indicated.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Luminescence resonance energy transfer between genetically encoded donor and acceptor for protein-protein interaction studies in the molecular chaperone HSP70/HSP90 complexes. Sci Rep (2018)

Knockdown of Hsp90α or Hsp90β with siRNA decreased the proportion of extracellular FN matrix.(A) Hs578T cells were transfected with a pool of siRNA against either Hsp90α (Hsp90α K/D) or Hsp90β (Hsp90β K/D). Scrambled non-targeting (NT) siRNA served as a negative control. After 48 hours, levels of Hsp90α and Hsp90β were analysed by immunoblotting with mouse anti human Hsp90α or Hsp90β primary antibodies respectively. Transfected cells were harvested and DOC-soluble and DOC-insoluble fractions separated. Levels of DOC-soluble and DOC-insoluble FN were analyzed by immunoblotting with mouse anti human FN primary antibody. Levels of GAPDH or Histone H3 were used to ensure equal sample loading (B) Relative densitometry of expression levels of Hsp90α and Hsp90β of NT and Hsp90 knockdown (K/D) transfected Hs578T cells, determined by Image J 1.43m. (C) Relative densitometry of levels of soluble and insoluble FN of NT and Hsp90 K/D transfected Hs578T cells, determined by Image J. (D) Transfected Hs578T cells were fixed and stained with mouse anti human FN followed by donkey anti mouse DyLight® 488 secondary antibody. Nuclei were stained with Hoechst 33342 (1 µg.ml−1). Images were captured using a Zeiss LSM 510 Meta laser scanning confocal microscope and analyzed using AxiovisionLE 4.7.1, Zeiss, Germany. Confocal microscopy images for each treatment were captured in triplicate and values in white represent the mean grey values (± standard deviation) for each treatment. Scale bars are equivalent to 20 µm. The data shown are from triplicate experiments. (E) Exogenous Hsp90β partially recovered the FN phenotype in Hsp90β knockdown cells. Adherent Hs578T cells were treated with siRNA against Hsp90β and then remained untreated or were treated with Hsp90β (100 ng.ml−1). Cells were fixed and stained for FN as described previously. Scale bars are equivalent to 100 µm. The average mean gray values and standard deviation from 5 different fields of view are indicated on the images in white text. Statistical analysis was performed by comparing siRNA treated cells to cells treated with siRNA and exogenous Hsp90β, using one way ANOVA with Bonferroni post-test. Images shown are representative of duplicate experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. PLoS One (2014)



Product Details
| Alternative Name |
HSP86, Heat shock protein 90α |
|---|---|
| Application |
Flow Cytometry, ICC, IHC, IP, WB |
| Application Notes |
Detects a band of ~90kDa by Western blot. |
| Clone |
9D2 |
| Formulation |
Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| Host |
Rat |
| Immunogen |
Purified Hsp90 isolated from human therapeutic orchiectomy specimens. |
| Isotype |
IgG2a |
| Purity Detail |
Protein G affinity purified. |
| Recommendation Dilutions/Conditions |
Flow Cytometry (1:100)Western Blot (1:1,000, colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rat ascites. |
| Species Reactivity |
Chicken, Human |
| UniProt ID |
P07900 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Integrative Multi-Omics Analyses Reveal Mechanisms of Resistance to Hsp90β-Selective Inhibition.: Mersich, I., Anik, E., et al.; Cancers (Basel) 17, 3488 (2025), Application(s): Western Blot, Abstract
- HSP90 N-terminal inhibition promotes mitochondria-derived vesicles related metastasis by reducing TFEB transcription via decreased HSP90AA1-HCFC1 interaction in liver cancer.: Liu, L., Zheng, Z., et al.; Autophagy 21, 639 (2025), Abstract
- ATP plays a structural role in Hsp90 function: Reidy, M., Masison, D. C., et al.; Nat. Commun. 16, 6710 (2025), Abstract
- ANXA2 (annexin A2) is crucial to ATG7-mediated autophagy, leading to tumor aggressiveness in triple-negative breast cancer cells: Koh, M., Lim, H., et al.; Autophagy 20, 659 (2024), Abstract
- Phosphorylation of the Hsp90 Co-Chaperone Hop Changes its Conformational Dynamics and Biological Function: M. Castelli, et al.; J. Mol. Biol. 435, 167931 (2023), Abstract
- Nucleotide exchange is sufficient for Hsp90 functions in vivo.: Reidy, M., Garzillo, K., et al.; Nat. Commun. 14, 2489 (2023), Abstract
- Hsf1 and the molecular chaperone Hsp90 support a ‘rewiring stress response’ leading to an adaptive cell size increase in chronic stress.: Maiti, S., Bhattacharya, K., et al.; Elife 12, (2023), Reactant(s): Human, Abstract
- Mutations in the Hsp90 N Domain Identify a Site that Controls Dimer Opening and Expand Human Hsp90α Function in Yeast.: Reidy, M., Masison, D. C., et al.; J. Mol. Biol. 432, 4673 (2020), Application(s): WB / Reactant(s): Rabbit, Abstract
- Avian uterine fluid proteome: Exosomes and biological processes potentially involved in sperm survival.: Riou, C., Brionne, A., et al.; Mol. Reprod. Dev. 87, 454 (2020), Application(s): WB, Abstract
- Dual Roles for Yeast Sti1/Hop in Regulating the Hsp90 Chaperone Cycle: Reidy, M., Kumar, S., et al.; Genetics 209, 1139 (2018), Abstract
- Functional and physical interaction between yeast Hsp90 and Hsp70.: Kravats, A. N., Hoskins, J. R., et al.; PNAS 115, E2210 (2018), Application(s): WB / Reactant(s): Saccharomyces cerevisiae, Abstract
- Luminescence resonance energy transfer between genetically encoded donor and acceptor for protein-protein interaction studies in the molecular chaperone HSP70/HSP90 complexes.: Bhattacharya, K., Bernasconi, L., et al.; Sci. Rep. 8, 2801 (2018), Application(s): WB / Reactant(s): Human, Abstract
- Extracellular Hsp90 and TGFβ regulate adhesion, migration and anchorage independent growth in a paired colon cancer cell line model: de la Mare, J. A., Jurgens, T., et al.; BMC Cancer 17, 202 (2017), Abstract
- An Hsp90 co-chaperone protein in yeast is functionally replaced by site-specific posttranslational modification in humans: Zuehlke, A. D., Reidy, M., et al.; Nat. Commun. 8, 15328 (2017), Abstract
- VPS34 stimulation of p62 phosphorylation for cancer progression.: Jiang, X., Zhang, Z., et al.; Oncogene 36, 6850 (2017), Application(s): WB, Abstract
- Secreted heat shock protein 90 promotes prostate cancer stem cell heterogeneity: K.D. Nolan, et al.; Oncotarget 8, 19323 (2017), Abstract — Full Text
- The association of Hsp90 expression induced by aspirin with anti-stress damage in chicken myocardial cells: X.H. Zhang, et al.; J. Vet. Sci. 17, 35 (2016), Application(s): Immunohistochemistry on heart tissue, Abstract — Full Text
- 4-hydroxybenzoic acid derivatives as HDAC6-specific inhibitors modulating microtubular structure and HSP90α chaperone activity against prostate cancer: C. Seidel, et al.; Biochem. Pharmacol. 99, 31 (2016), Application(s): Western blot, Immunoprecipitation, Abstract
- Histone deacetylase inhibitor panobinostat induces calcineurin degradation in multiple myeloma.: Maru, Y., Ishibashi, M., et al.; JCI Insight 1, e85061 (2016), Application(s): IP / Reactant(s): Human, Abstract
- Histone deacetylase inhibitors prevent pulmonary endothelial hyperpermeability and acute lung injury by regulating heat shock protein 90 function.: Catravas, J. D., Cherian-Shaw, M., et al.; Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1410 (2015), Reactant(s): Mouse, Abstract
- Phosphorylated and Unphosphorylated Serine 13 of CDC37 Stabilize Distinct Interactions Between its Client and HSP90 Binding Domains: W. Liu & R. Landgraf; Biochemistry 54, 1493 (2015), Application(s): Western blot using human BT474 breast cancer cell lysates, Abstract — Full Text
- KU675, a Concomitant Heat Shock Protein Inhibitor of Hsp90 and Hsc70 that Manifests Isoform Selectivity for Hsp90α in Prostate Cancer Cells: W. Liu, et al.; Mol. Pharmacol. 88, 121 (2015), Application(s): Western Blot, Abstract — Full Text
- Expression of Hsp90α and cyclin B1 were related to prognosis of esophageal squamous cell carcinoma and keratin pearl formation: T. Huang, et al.; Int. J. Clin. Exp. Pathol. 7, 1544 (2014), Application(s): IHC of human esophageal squamous cell carcinoma, Abstract — Full Text
- Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells: M. Hunter, et al.; PLoS One 9, e86842 (2014), Application(s): WB, IP of human breast cancer cells, Abstract — Full Text
- Human TRiC complex purified from HeLa cells contains all eight CCT subunits and is active in vitro: K. Knee, et al.; Cell Stress Chaperones 18, 137 (2013), Application(s): WB of human TRiC from HeLa cell lysate, Abstract — Full Text
- HSP90 and HSP70 proteins are essential for stabilization and activation of WASF3 metastasis-promoting protein: Y. Teng, et al.; J. Biol. Chem. 287, 10051 (2012), Application(s): Western blot using human immunoprecipitated PC-3 cell lysates, Abstract — Full Text
- A potentially common peptide target in secreted heat shock protein-90α for hypoxia-inducible factor-1α-positive tumors: D. Sahu, et al.; Mol. Biol. Cell 23, 602 (2012), Application(s): Western blot using human HBL-100, HKC, MC, HDF, SCC-12, M-24, A431 and MDA-MB-231 cell lysates, Abstract — Full Text
- Differential proteomic analysis of human erythroblasts undergoing apoptosis induced by epo-withdrawal: S. Pellegrin, et al.; PLoS One 7, e38356 (2012), Application(s): WB of human erythroblasts, Abstract — Full Text
- Heat shock protein 90α (HSP90α), a substrate and chaperone of DNA-PK necessary for the apoptotic response: S. Solier, et al.; Proc. Natl. Acad. Sci. 109, 12866 (2012), Application(s): WB, IF of human colon, cervical, and leukemic carcinomas, Abstract — Full Text
- Association of hsp90 to the hTERT promoter is necessary for hTERT expression in human oral cancer cells: M. Kang, et al.; Carcinogenesis 29, 2425 (2008), Application(s): WB, IP, ChIP using human cell lysates, Abstract
- Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK+ CD30+ lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin: P. Bonvini, et al.; Cancer Res. 62, 1559 (2002), Application(s): IP, WB using human samples, Abstract
- Expression of hsp90 in the human kidney and in proximal tubule cells exposed to heat, sodium arsenite and cadmium chloride: S. Somji, et al.; Toxicol. Lett. 133, 241 (2002), Application(s): IHC, WB using human samples, Abstract
- A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth: S.L. Nishimura, et al.; Cancer Res. 60, 7084 (2000), Application(s): WB using human samples, Abstract
- Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies: B.T. Lai, et al.; Mol. Cell. Biol. 4, 2802 (1984), Application(s): IP, ICC using human samples, Abstract
Related Products
HSP90 (human), (native)
ADI-SPP-770
Native human Hsp90, a highly conserved cytosolic chaperone that, in cooperation with co-chaperones, stabilizes client proteins including kinases, transcription factors, and hormone receptors, and is essential for ATP-dependent folding and activation.
| Alternative Name | HSP86, Heat shock protein 90 |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in HeLa cells. |
HSP90α (human), (recombinant)
ADI-SPP-776
Recombinant human Hsp90α, a cytosolic chaperone that cooperates with co-chaperones to stabilize and fold client proteins including kinases, transcription factors, and steroid hormone receptors in an ATP-dependent manner.

| Alternative Name | HSP86, Heat shock protein 90α |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
Last modified: August 6, 2025
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?