The Hsp90 family of heat shock proteins represents one of the most abundantly expressed and highly conserved families of cellular chaperones whose expression can be upregulated under conditions of cellular stress, and includes cytoplasmic (Hsp90-alpha/beta), ER (grp94), and mitochondrial (TRAP1) localized members. Structurally, Hsp90 is characterized by an N-terminal ATP-binding domain, a medial substrate-binding domain, and a C-terminal dimerization motif. Hsp90 dimers function in cooperation with cochaperones (e.g. Hsp40, Hsp70, Hop, p23) to stabilize a multitude of client protein substrates, including steroid hormone receptors, protein kinases, and transcription factors. The essential binding and hydrolysis of ATP by Hsp90 is inhibited by ansamycin drugs (e.g. geldanamycin, 17-AAG) which occupy the N-terminal Hsp90 nucleotide-binding pocket. Many Hsp90 client proteins such as erbB2/Her-2, c-raf, bcr-abl, p53, and hTERT, are members of well characterized oncogenic pathways, making Hsp90 inhibitors useful anticancer agents.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
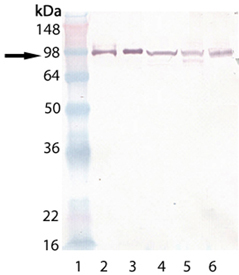
Western blot analysis of HSP90, pAb (Prod. No. ADI-SPA-836): Lane 1: MW marker, Lane 2:HSP90α (human), (rec) (Prod. No. ADI-SPP-776), Lane 3: HSP90β (human), (rec) (Prod. No. ADI-SPP-777), Lane 4: HeLa (heat shocked) (Prod. No. ADI-LYC-HL101), Lane 5: 3T3 (heat shocked) (Prod. No. ADI-LYC-3T101),Lane 6: PC-12 (heat shocked) (Prod. No. ADI-LYC-PC101).
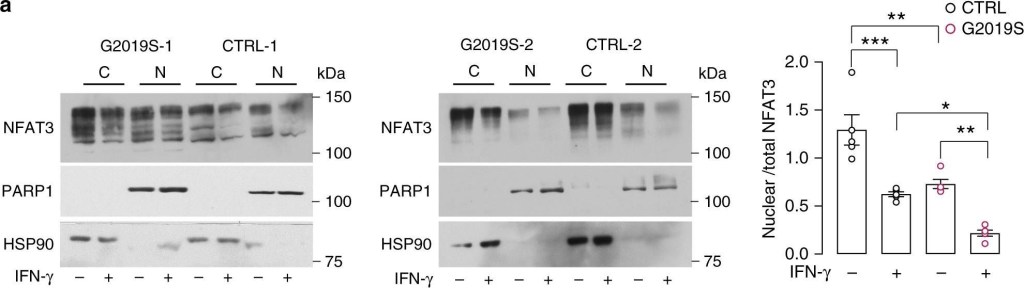
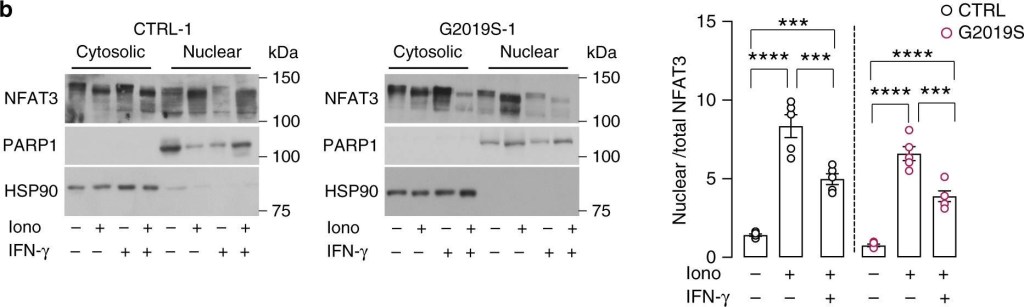
LRRK2 inhibits nuclear NFAT3 shuttling in human neurons.a Representative Western blots of NFAT3 in nuclear (N) and cytosolic (C) fractions of LRRK2 G2019S neurons and isogenic controls (left and middle panel). Treatment with 200 IU/mL IFN-γ is indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0003, **P = 0.0015, 0.0035 in sequence, *P = 0.0226; n = 5 independent experiments). b Representative Western blots of NFAT3 in N and C fractions of control (left panel) and isogenic LRRK2 G2019S neurons (middle panel). Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001, ***P = 0.0005, 0.0008, 0.0002 in sequence; n = 5 independent experiments). c Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in LRRK2 G2019S neurons and isogenic controls. Treatments with 200 IU/mL IFN-γ are indicated (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0009, *P = 0.0421; n = 5 independent experiments). d Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in control iPSC-derived neurons upon treatment with 200 IU/mL IFN-γ for 24 h, with and without treatment with a proteasome inhibitor (MG132, 20 nM for 24 h) (mean ± SEM, one-way ANOVA, Bonferroni post hoc, *P = 0.0399, 0.0437 in sequence; n = 3 independent experiments). e Representative Western blots of NFAT3 in nuclear and cytosolic fractions of LRRK2 KO neurons. Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001; n = 5 independent experiments).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun (2020)
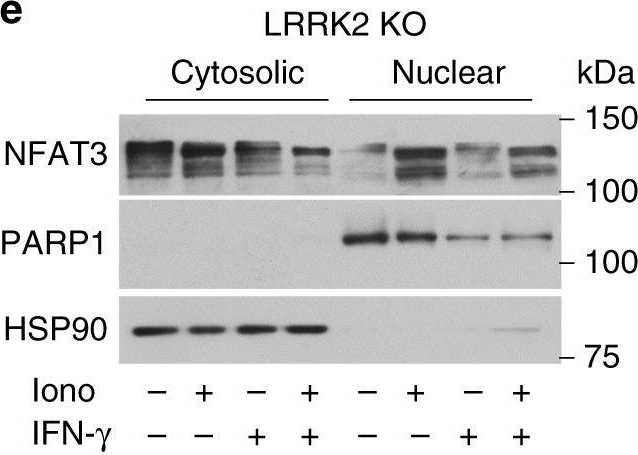
LRRK2 inhibits nuclear NFAT3 shuttling in human neurons.a Representative Western blots of NFAT3 in nuclear (N) and cytosolic (C) fractions of LRRK2 G2019S neurons and isogenic controls (left and middle panel). Treatment with 200 IU/mL IFN-γ is indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0003, **P = 0.0015, 0.0035 in sequence, *P = 0.0226; n = 5 independent experiments). b Representative Western blots of NFAT3 in N and C fractions of control (left panel) and isogenic LRRK2 G2019S neurons (middle panel). Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001, ***P = 0.0005, 0.0008, 0.0002 in sequence; n = 5 independent experiments). c Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in LRRK2 G2019S neurons and isogenic controls. Treatments with 200 IU/mL IFN-γ are indicated (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0009, *P = 0.0421; n = 5 independent experiments). d Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in control iPSC-derived neurons upon treatment with 200 IU/mL IFN-γ for 24 h, with and without treatment with a proteasome inhibitor (MG132, 20 nM for 24 h) (mean ± SEM, one-way ANOVA, Bonferroni post hoc, *P = 0.0399, 0.0437 in sequence; n = 3 independent experiments). e Representative Western blots of NFAT3 in nuclear and cytosolic fractions of LRRK2 KO neurons. Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001; n = 5 independent experiments).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun (2020)
LRRK2 inhibits nuclear NFAT3 shuttling in human neurons.a Representative Western blots of NFAT3 in nuclear (N) and cytosolic (C) fractions of LRRK2 G2019S neurons and isogenic controls (left and middle panel). Treatment with 200 IU/mL IFN-γ is indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0003, **P = 0.0015, 0.0035 in sequence, *P = 0.0226; n = 5 independent experiments). b Representative Western blots of NFAT3 in N and C fractions of control (left panel) and isogenic LRRK2 G2019S neurons (middle panel). Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 in LRRK2 G2019S iPSC-derived neurons and isogenic controls (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001, ***P = 0.0005, 0.0008, 0.0002 in sequence; n = 5 independent experiments). c Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in LRRK2 G2019S neurons and isogenic controls. Treatments with 200 IU/mL IFN-γ are indicated (mean ± SEM, two-way ANOVA, Bonferroni post hoc, ***P = 0.0009, *P = 0.0421; n = 5 independent experiments). d Representative Western blot (left) and quantification (right) of NFAT3 from whole-cell extracts in control iPSC-derived neurons upon treatment with 200 IU/mL IFN-γ for 24 h, with and without treatment with a proteasome inhibitor (MG132, 20 nM for 24 h) (mean ± SEM, one-way ANOVA, Bonferroni post hoc, *P = 0.0399, 0.0437 in sequence; n = 3 independent experiments). e Representative Western blots of NFAT3 in nuclear and cytosolic fractions of LRRK2 KO neurons. Treatments with 1 μM ionomycin for 30 min and 200 IU/mL IFN-γ for 24 h are indicated. On the right, quantification of nuclear to total (N and C fraction) NFAT3 (mean ± SEM, one-way ANOVA, Bonferroni post hoc, ****P < 0.0001; n = 5 independent experiments).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun (2020)




Product Details
| Alternative Name |
Heat shock protein 90 |
|---|---|
| Application |
IHC (PS), WB |
| Application Notes |
Detects a band of ~90kDa by Western blot. |
| Formulation |
Liquid. In PBS containing 50% glycerol and 0.09% sodium azide. |
| Host |
Rabbit |
| Immunogen |
Synthetic peptide corresponding to aa 250-325 of human Hsp90. |
| Purity Detail |
Protein A affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, Colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rabbit serum. |
| Species Reactivity |
Human, Mouse, Rabbit, Rat |
| UniProt ID |
P07900 (HSP90α), P08238 (HSP90β) |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Inhibition of VEGFR-3 by SAR131675 decreases renal inflammation and lymphangiogenesis in the murine lupus nephritis model: Wang, T., Li, W., et al.; Cell Death Discov. 11, 320 (2025), Abstract
- Cannabidiol reshapes the gut microbiome to promote endurance exercise in mice: Chen, S., Lee, Y. B., et al.; Exp. Mol. Med. 57, 489 (2025), Abstract
- Interaction between p21-activated kinase 4 and β-catenin as a novel pathway for PTH-dependent osteoblast activation: Shen, C., Oh, H. R., et al.; J. Cell. Physiol. 239, e31245 (2024), Abstract
- Cannabidiol Inhibits IgE-Mediated Mast Cell Degranulation and Anaphylaxis in Mice: Yang, X., Lee, D., et al.; Mol. Nutr. Food Res. 68, e2300136 (2024), Abstract
- Phosphorylation of PBX2, a novel downstream target of mTORC1, is determined by GSK3 and PP1: R. Wada, et al.; J. Biochem. 173, 129 (2023), Abstract
- NAD+-boosting molecules suppress mast cell degranulation and anaphylactic responses in mice: H.W. Kim, et al.; Theranostics 12, 3316 (2022), Application(s): WB, Abstract
- Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression.: Bae, E. J., Song, M. Y., et al.; Nat. Commun. 13, 1808 (2022), Application(s): WB / Reactant(s): Human, Abstract
- Limonium tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation.: Lee, Y. G., Song, M. Y., et al.; Nutrients 14, (2022), Application(s): WB / Reactant(s): Mouse, Abstract
- Heterogeneous nuclear ribonucleoprotein K is overexpressed in acute myeloid leukemia and causes myeloproliferation in mice via altered Runx1 splicing.: Aitken, M. J. L., Malaney, P., et al.; NAR Cancer 4, zcac039 (2022), Application(s): WB, Abstract
- Heat shock factor 1 (HSF1) cooperates with estrogen receptor α (ERα) in the regulation of estrogen action in breast cancer cells.: Kimmel, M., Stokowy, T., et al.; Elife 10, (2021), Application(s): ICC, ICC-IF, PLA, WB / Reactant(s): Human, Abstract
- Heat Shock Factor 1 (HSF1) as a new tethering factor for ESR1 supporting its action in breast cancer: Kimmel, M., Stokowy, T., et al.; bioRxiv , (2021), Application(s): WB / Reactant(s): Human
- Heterogeneous nuclear ribonucleoprotein K is overexpressed in acute myeloid leukemia and causes myeloproliferative disease in mice via altered i>Runx1/i> splicing: Aitken, M. J. L., Malaney, P., et al.; bioRxiv , (2021)
- Interferon-γ signaling synergizes with LRRK2 in human neurons and microglia: De Cicco, S., Ivanyuk, D., et al.; bioRxiv , (2020)
- Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells.: Panagiotakopoulou, V., Ivanyuk, D., et al.; Nat. Commun. 11, 5163 (2020), Application(s): WB / Reactant(s): Human, Abstract
- ERRγ suppression by Sirt6 alleviates cholestatic liver injury and fibrosis: Hao, L., Bang, I. H., et al.; JCI Insight 5, (2020), Application(s): WB, Abstract
- HTLV-1 viral oncogene HBZ drives bone destruction in adult T cell leukemia: J. Xiang, et al.; JCI Insight 4, e128713 (2019), Abstract — Full Text
- The O-GlcNAc Transferase Intellectual Disability Mutation L254F Distorts the TPR Helix: M. Gundogdu, et al.; Cell Chem. Biol. 25, 513 (2018), Abstract
- A Novel Class of Hsp90 C-Terminal Modulators Have Pre-Clinical Efficacy in Prostate Tumor Cells Without Induction of a Heat Shock Response: Armstrong, H. K., Koay, Y. C., et al.; Prostate 76, 1546 (2016), Abstract
- Hitting a Moving Target: How Does an N-Methyl Group Impact Biological Activity?: Koay, Y. C., Richardson, N. L., et al.; ChemMedChem 11, 881 (2016), Abstract
- CHCHD10 mutations promote loss of mitochondrial cristae junctions with impaired mitochondrial genome maintenance and inhibition of apoptosis.: Genin, E. C., Plutino, M., et al.; EMBO Mol. Med. 8, 58 (2016), Abstract
- Expression of Heat Shock Proteins (HSPs) in Aged Skeletal Muscles Depends on the Frequency and Duration of Exercise Training.: Lee, Y. H., Yi, H. K., et al.; J. Sports Sci. Med. 14, 347 (2015), Application(s): WB / Reactant(s): Rat, Abstract
- Identification of heat-shock protein 90 beta in Japanese encephalitis virus-induced secretion proteins.: Wu, Y. P., Hung, C. Y., et al.; J. Gen. Virol. 92, 2803 (2011), Application(s): WB / Reactant(s): Hamster, Abstract
Related Products

| Application | ELISA, WB |
|---|---|
| Host | Goat |
| Species Reactivity | Rabbit |
HSP90α (human), (recombinant)
ADI-SPP-776
Recombinant human Hsp90α, a cytosolic chaperone that cooperates with co-chaperones to stabilize and fold client proteins including kinases, transcription factors, and steroid hormone receptors in an ATP-dependent manner.
| Alternative Name | HSP86, Heat shock protein 90α |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?