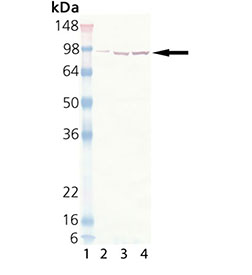
The 90kDa molecular chaperone family includes 90 kDa heat shock protein Hsp90 and 94 kDa glucose-regulated protein grp94, both major molecular chaperones of the cytosol and the endoplasmic reticulum. Mammalian cells contain isoforms Hsp90α and Hsp90β, encoded by separate genes. The amino acid sequences of human and yeast Hsp90-alpha are 85% and 90% homologous to those of Hsp90β , respectively. All known members of the Hsp90 protein family are highly conserved, especially in the N-terminal and C-terminal regions containing independent chaperone sites with different substrate specificity. These ubiquitous and highly conserved proteins account for 1-2% of all cellular proteins in most cells. Hsp90 functions as part of the cell’s powerful network of chaperones to fight the deleterious consequences of protein unfolding caused by non-physiological conditions. In the absence of stress, however, Hsp90 provides a necessary component of such fundamental cellular processes as hormone signaling and cell cycle control. In this context, researchers identified key regulatory proteins as substrates of Hsp90, including steroid receptors, cell cycle kinases involved in signal transduction, and p53. Hsp90 may act as a capacitor for morphological evolution by buffering widespread variation, potentially affecting morphogenic pathways. When temperature and other stress factors compromise Drosophila Hsp90 buffering, cryptic variant expression occurs, and selection can lead to the continued expression of these traits even after Hsp90 function is restored.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?