The Hsp90 family of heat shock proteins represents one of the most abundantly expressed and highly conserved families of cellular chaperones whose expression can be upregulated under conditions of cellular stress, and includes cytoplasmic (Hsp90-alpha/beta), ER (grp94), and mitochondrial (TRAP1) localized members. Structurally, Hsp90 is characterized by an N-terminal ATP-binding domain, a medial substrate-binding domain, and a C-terminal dimerization motif. Hsp90 dimers function in cooperation with cochaperones (e.g. Hsp40, Hsp70, Hop, p23) to stabilize a multitude of client protein substrates, including steroid hormone receptors, protein kinases, and transcription factors. The essential binding and hydrolysis of ATP by Hsp90 is inhibited by ansamycin drugs (e.g. geldanamycin, 17-AAG) which occupy the N-terminal Hsp90 nucleotide-binding pocket. Many Hsp90 client proteins such as erbB2/Her-2, c-raf, bcr-abl, p53, and hTERT, are members of well characterized oncogenic pathways, making Hsp90 inhibitors useful anticancer agents.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
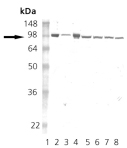
Western blot analysis of HSP90: Lane 1: MW marker, Lane 2: HSP90 native protein (ADI-SPP-770), Lane 3: HSP90b recombinant protein (Prod. No. ADI-SPP-772), Lane 4: HSP90alpha recombinant protein (Prod. No. ADI-SPP-776), Lane 5: HeLa (HS), Lane 6: L-929 (HS), Lane 7: Rat-2 (HS), Lane 8:RK-13 (HS).
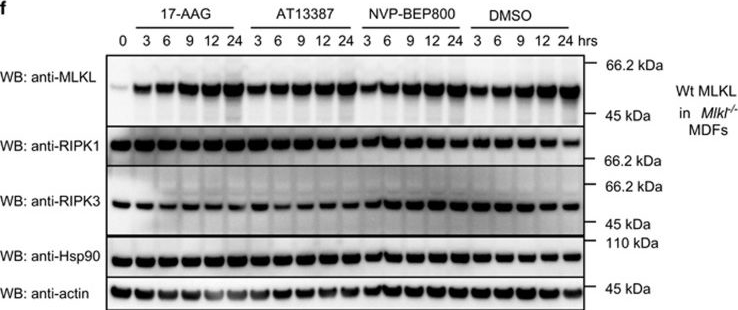
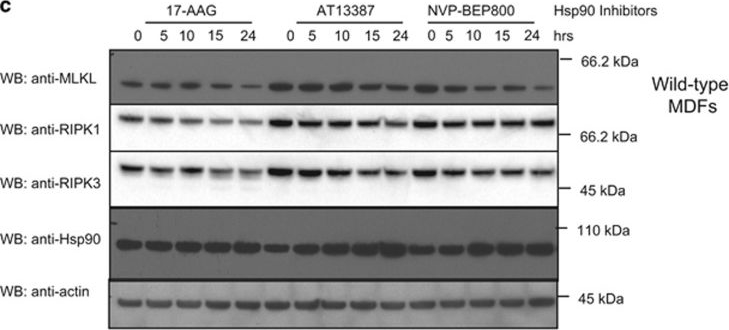
Hsp90 inhibition affects MLKL activity. (a–c) Wild-type (a), Mlkl−/− (b) or Ripk3−/− (c) MDFs were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then expression of MLKL(1–180) was induced using 50 ng/ml doxycycline. After 24 h, PI uptake was measured using flow cytometry. Two experiments were performed using three independent cell lines (n=6). (d) Mlkl−/−Ripk3−/− MDFs were pretreated with HSP90 inhibitors as described above, then expression of MLKL S345D was induced using 50 ng/ml doxycycline. After 24 h, PI uptake was measured using flow cytometry. Two experiments were performed using three independent cell lines (n=6). (e and f) Cells were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then expression of MLKL S345D in Mlkl−/−Ripk3−/− double knockout MDFs (e) or endogenous MLKL in Mlkl−/− MDFs (f) was induced using 50 ng/ml doxycycline. Treatment was performed over 24 h at the times shown, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
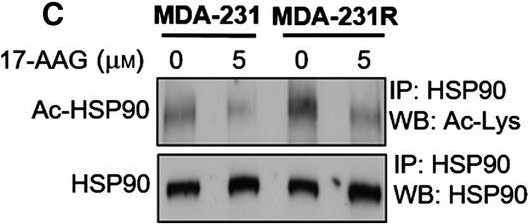
Altered NQO1 levels, HDAC family member expression and altered acetylation status in 17‐AAG‐resistant cell lines. Analysis of parental and resistant cell lines demonstrated altered expression levels of a number of molecules. Semiquantitative PCR demonstrated that the expression levels of NQO1 in resistant MDA‐435 cells were decreased when compared with parental cells, while no alteration was noted between MDA‐231 and MDA‐231R cell lines (A). Western blot analysis of parental and resistant MDA‐231 total cell lysates examining levels of HDAC family members in the presence and absence of 17‐AAG for a period of 24 h (B). Analysis of acetylated HSP90 by immunoprecipitation of HSP90 and western blot analysis with antiacetylated lysine antibody of total cell lysates of parental and resistant MDA‐231 cells treated with and without 17‐AAG demonstrated increased acetylated HSP90 (C). Analysis of acetylation of Grp94 (D) and Trap1 (E) by immunoprecipitation and western blot analysis of MDA‐231 and MDA‐231R total cell lysates demonstrated no alteration in acetylation status. Acetylated lysine residue was detected by western blotting. Western blot analysis of acetylated histone 3 in parental and resistant MDA‐231 cells treated with and without 17‐AAG demonstrated decreased nuclear acetylation (F).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors. Mol Oncol (2017)
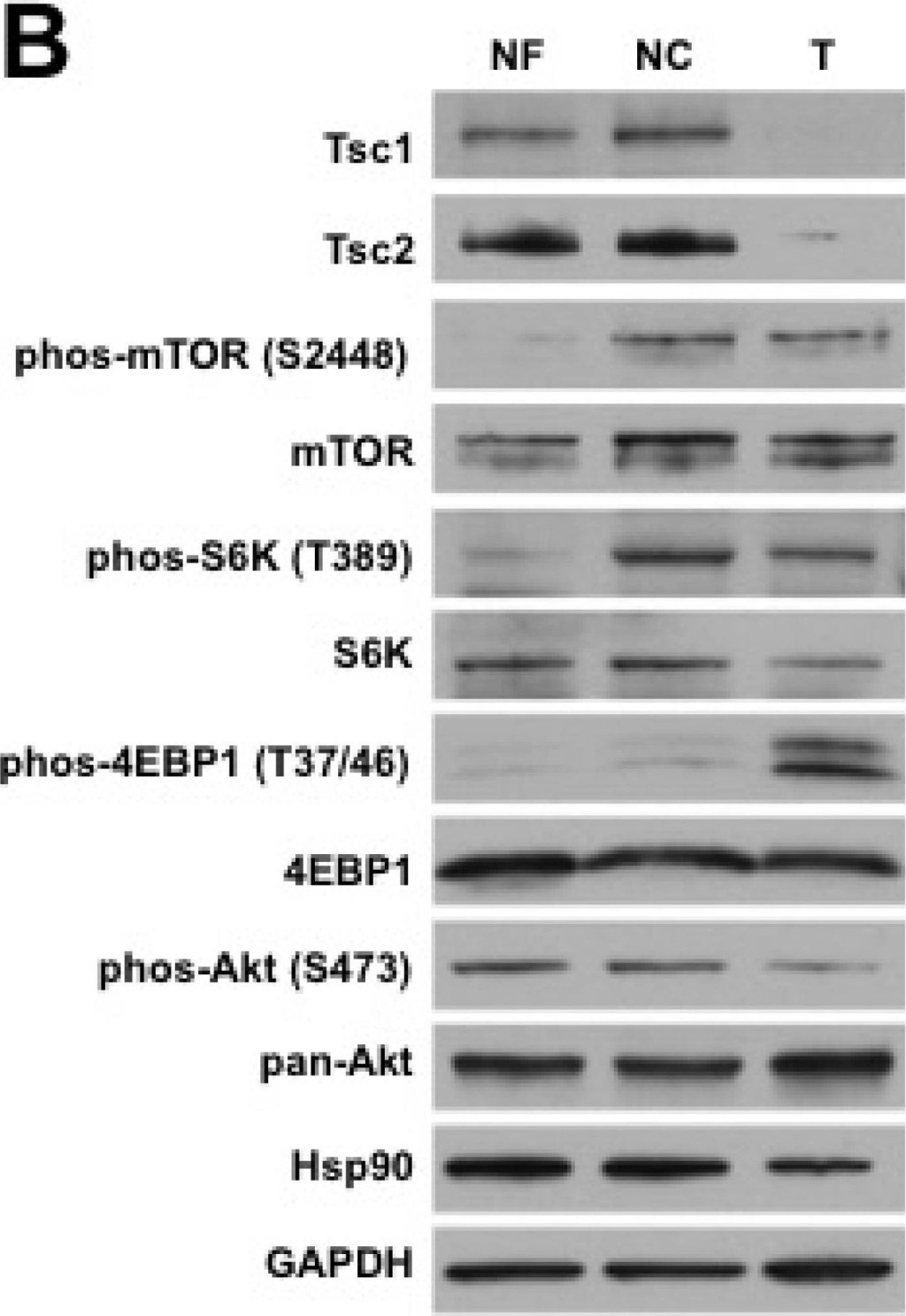
Sporadic renal AML demonstrates somatic loss of Tsc1/2 expression(A) Protein was extracted from adjacent normal far (NF) and close (NC) kidney and tumor (T). Expression of FLCN was examined by immunoblotting. GAPDH was used as a loading control. Short (SE) and long (LE) exposure of the radiographic film. (B) Protein was extracted from adjacent normal far (NF) and close (NC) kidney and tumor (T). Expression of Tsc1/2 and mTOR pathway components was examined by immunoblotting. GAPDH was used as a loading control.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Sporadic renal angiomyolipoma in a patient with Birt-Hogg-Dubé: chaperones in pathogenesis. Oncotarget (2018)
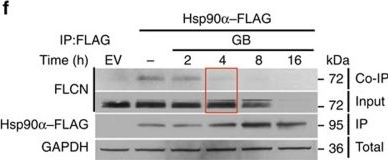
Folliculin is a new client of Hsp90.(a) FLAG–FLCN was expressed and isolated from HEK293 cells. Profile of interacting proteins determined by MALDI–time of flight. Red nodes represent chaperones and co-chaperones, blue nodes are chaperonins and green nodes are splicing factors and ribosomal proteins. (b) FLCN was isolated from HEK293 cell lysates using anti-FLCN or IgG (control) and immunoblotted with indicated antibodies to confirm protein interactions. (c) HEK293 cells were transiently transfected with FLAG–FLCN or empty vector control (EV), immunoprecipitated and immunoblotted with indicated antibodies to confirm interacting proteins. (d) HEK293 cells were treated with 10 μM of the Hsp70 inhibitor JG-98 at the indicated time points. FLCN protein stability in soluble and insoluble fraction was assessed by immunoblotting. (e) HEK293 cells were treated with 1 μM GB at the indicated time points. FLCN protein stability was assessed by immunoblotting. Akt and Phospho-S473-Akt were used as positive controls. (f) Hsp90α–FLAG was transiently expressed in HEK293 cells. Cells were treated with 1 μM GB for the indicated times. Hsp90α–FLAG was immunoprecipitated and co-IP of FLCN was examined by immunoblotting. (g) HEK293 cells were treated with 50 nM of the proteasome inhibitor bortezomib (BZ) for the indicated times. FLCN protein levels were evaluated at the indicated time points by immunoblotting (upper blots). HEK293 cells were also treated with 1 μM GB for 1 h before addition of 50 nM BZ. Immunoblotting was used to evaluate the FLCN level for the indicated time points (lower blots). (h) Empty vector (EV) or FLAG–FLCN was used to transiently transfect HEK293 cells for 24 h then treated for 4 h with either 50 nm BZ or 1 μM GB. FLAG–FLCN was immunoprecipitated and ubiquitination was examined by immunoblotting with a pan-anti-ubiquitin antibody.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
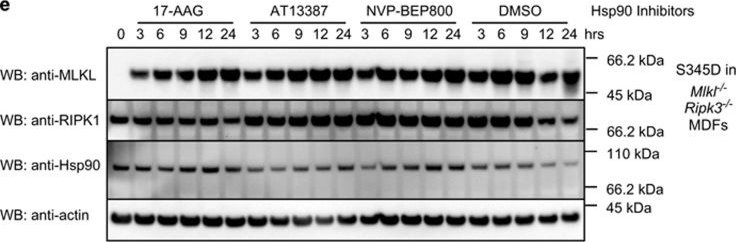
Hsp90 inhibition affects MLKL activity. (a–c) Wild-type (a), Mlkl−/− (b) or Ripk3−/− (c) MDFs were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then expression of MLKL(1–180) was induced using 50 ng/ml doxycycline. After 24 h, PI uptake was measured using flow cytometry. Two experiments were performed using three independent cell lines (n=6). (d) Mlkl−/−Ripk3−/− MDFs were pretreated with HSP90 inhibitors as described above, then expression of MLKL S345D was induced using 50 ng/ml doxycycline. After 24 h, PI uptake was measured using flow cytometry. Two experiments were performed using three independent cell lines (n=6). (e and f) Cells were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then expression of MLKL S345D in Mlkl−/−Ripk3−/− double knockout MDFs (e) or endogenous MLKL in Mlkl−/− MDFs (f) was induced using 50 ng/ml doxycycline. Treatment was performed over 24 h at the times shown, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
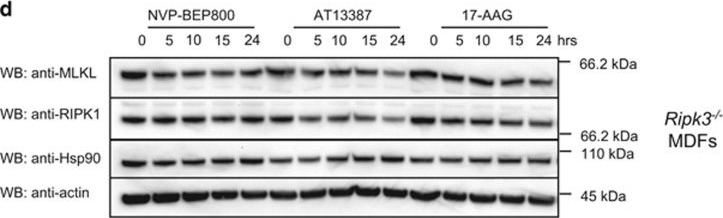
MLKL levels are modestly reduced by Hsp90 inhibition. (a) MDFs were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, propidium iodide (PI) uptake was measured using flow cytometry. Each data point represents results from one of three independent cell lines tested in two experiments and solid bar indicates average (n=6). (b) U937 cells were pretreated for 1 h with AT13387 (2 μM), NVP-BEP800 (1 μM), 17-AAG (500 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, PI uptake was measured using flow cytometry. Each data point represents results from the U937 cell line tested in three independent experiments and solid bar indicates average (n=3). (c and d) Wild-type (c) or Ripk3−/− (d) MDFs were treated with AT13387 (1 μM), NVP-BEP800 (125 nM) or 17-AAG (250 nM) for the indicated times, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. (e) U937 cells were treated with AT13387 (2 μM), NVP-BEP800 (1 μM) or 17-AAG (500 nM) over a 24-h time course, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. *Represents non-specific band at ~110 kDa
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
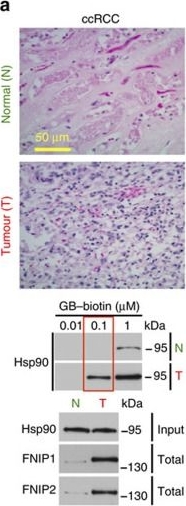
High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
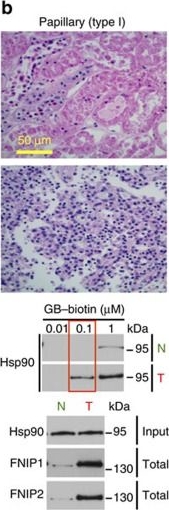
High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
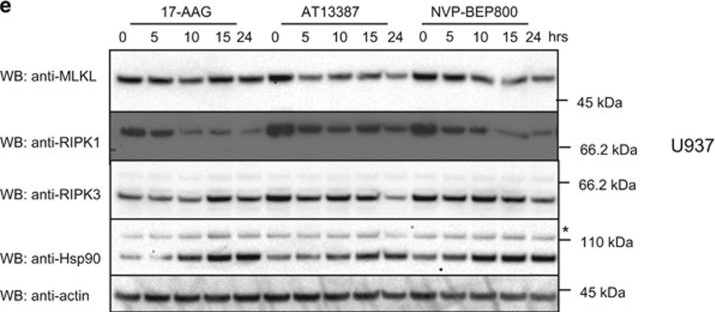
MLKL levels are modestly reduced by Hsp90 inhibition. (a) MDFs were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, propidium iodide (PI) uptake was measured using flow cytometry. Each data point represents results from one of three independent cell lines tested in two experiments and solid bar indicates average (n=6). (b) U937 cells were pretreated for 1 h with AT13387 (2 μM), NVP-BEP800 (1 μM), 17-AAG (500 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, PI uptake was measured using flow cytometry. Each data point represents results from the U937 cell line tested in three independent experiments and solid bar indicates average (n=3). (c and d) Wild-type (c) or Ripk3−/− (d) MDFs were treated with AT13387 (1 μM), NVP-BEP800 (125 nM) or 17-AAG (250 nM) for the indicated times, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. (e) U937 cells were treated with AT13387 (2 μM), NVP-BEP800 (1 μM) or 17-AAG (500 nM) over a 24-h time course, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. *Represents non-specific band at ~110 kDa
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
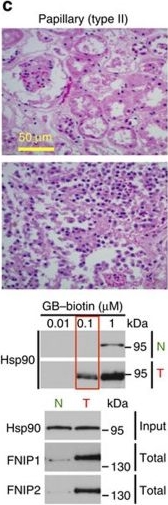
High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
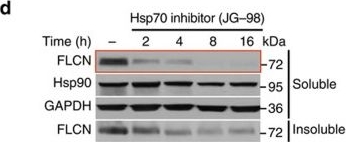
Folliculin is a new client of Hsp90.(a) FLAG–FLCN was expressed and isolated from HEK293 cells. Profile of interacting proteins determined by MALDI–time of flight. Red nodes represent chaperones and co-chaperones, blue nodes are chaperonins and green nodes are splicing factors and ribosomal proteins. (b) FLCN was isolated from HEK293 cell lysates using anti-FLCN or IgG (control) and immunoblotted with indicated antibodies to confirm protein interactions. (c) HEK293 cells were transiently transfected with FLAG–FLCN or empty vector control (EV), immunoprecipitated and immunoblotted with indicated antibodies to confirm interacting proteins. (d) HEK293 cells were treated with 10 μM of the Hsp70 inhibitor JG-98 at the indicated time points. FLCN protein stability in soluble and insoluble fraction was assessed by immunoblotting. (e) HEK293 cells were treated with 1 μM GB at the indicated time points. FLCN protein stability was assessed by immunoblotting. Akt and Phospho-S473-Akt were used as positive controls. (f) Hsp90α–FLAG was transiently expressed in HEK293 cells. Cells were treated with 1 μM GB for the indicated times. Hsp90α–FLAG was immunoprecipitated and co-IP of FLCN was examined by immunoblotting. (g) HEK293 cells were treated with 50 nM of the proteasome inhibitor bortezomib (BZ) for the indicated times. FLCN protein levels were evaluated at the indicated time points by immunoblotting (upper blots). HEK293 cells were also treated with 1 μM GB for 1 h before addition of 50 nM BZ. Immunoblotting was used to evaluate the FLCN level for the indicated time points (lower blots). (h) Empty vector (EV) or FLAG–FLCN was used to transiently transfect HEK293 cells for 24 h then treated for 4 h with either 50 nm BZ or 1 μM GB. FLAG–FLCN was immunoprecipitated and ubiquitination was examined by immunoblotting with a pan-anti-ubiquitin antibody.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
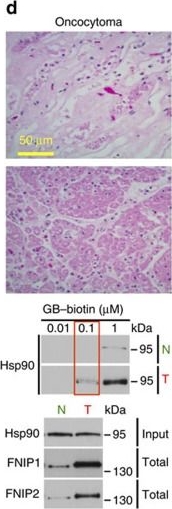
High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
MLKL levels are modestly reduced by Hsp90 inhibition. (a) MDFs were pretreated for 1 h with AT13387 (1 μM), NVP-BEP800 (125 nM), 17-AAG (250 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, propidium iodide (PI) uptake was measured using flow cytometry. Each data point represents results from one of three independent cell lines tested in two experiments and solid bar indicates average (n=6). (b) U937 cells were pretreated for 1 h with AT13387 (2 μM), NVP-BEP800 (1 μM), 17-AAG (500 nM) or DMSO, then necroptosis was induced with TSQ. After 24 h, PI uptake was measured using flow cytometry. Each data point represents results from the U937 cell line tested in three independent experiments and solid bar indicates average (n=3). (c and d) Wild-type (c) or Ripk3−/− (d) MDFs were treated with AT13387 (1 μM), NVP-BEP800 (125 nM) or 17-AAG (250 nM) for the indicated times, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. (e) U937 cells were treated with AT13387 (2 μM), NVP-BEP800 (1 μM) or 17-AAG (500 nM) over a 24-h time course, then cell lysates analysed with western blotting using the indicated antibodies. Data are representative of three independent experiments. *Represents non-specific band at ~110 kDa
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)

High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
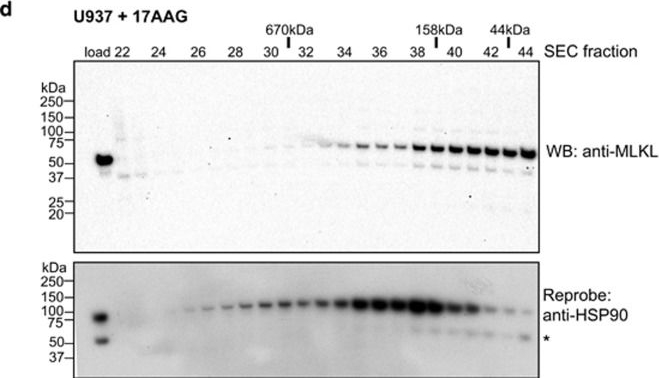
MLKL transiently interacts with HSP90 via the Cdc37 co-chaperone. (a and b) Mlkl−/−Ripk3−/− MDFs stably transduced with a lentiviral construct encoding S345D MLKL were untreated, treated with transfection reagent only, or transfected with scrambled or Cdc37 siRNA pools. Cdc37 knockdown was observed in Cdc37 siRNA-treated cells relative to untreated, transfection reagent and scrambled siRNA-treated controls by western blot (a). Only Cdc37 siRNA knockdown conferred protection from S345D MLKL-mediated death (b). (c and d) Lysates of U937 cells incubated with DMSO (c) or 17-AAG (500 nM, d) were resolved by Superose-6 10/300 size-exclusion chromatography (SEC). Fractions were subjected to SDS-PAGE and western blotted for MLKL (upper panels) before reprobing for HSP90 (lower; * corresponds to residual signal from MLKL blots). The SEC fraction number is shown above the blots along with the elution position of molecular weight standards
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
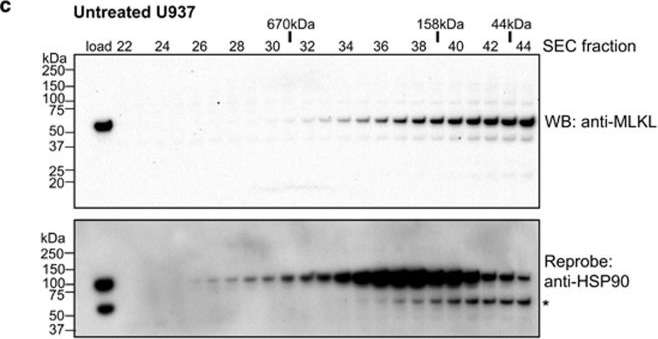
MLKL transiently interacts with HSP90 via the Cdc37 co-chaperone. (a and b) Mlkl−/−Ripk3−/− MDFs stably transduced with a lentiviral construct encoding S345D MLKL were untreated, treated with transfection reagent only, or transfected with scrambled or Cdc37 siRNA pools. Cdc37 knockdown was observed in Cdc37 siRNA-treated cells relative to untreated, transfection reagent and scrambled siRNA-treated controls by western blot (a). Only Cdc37 siRNA knockdown conferred protection from S345D MLKL-mediated death (b). (c and d) Lysates of U937 cells incubated with DMSO (c) or 17-AAG (500 nM, d) were resolved by Superose-6 10/300 size-exclusion chromatography (SEC). Fractions were subjected to SDS-PAGE and western blotted for MLKL (upper panels) before reprobing for HSP90 (lower; * corresponds to residual signal from MLKL blots). The SEC fraction number is shown above the blots along with the elution position of molecular weight standards
Image collected and cropped by CiteAb under a CC-BY license from the following publication: HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis (2016)
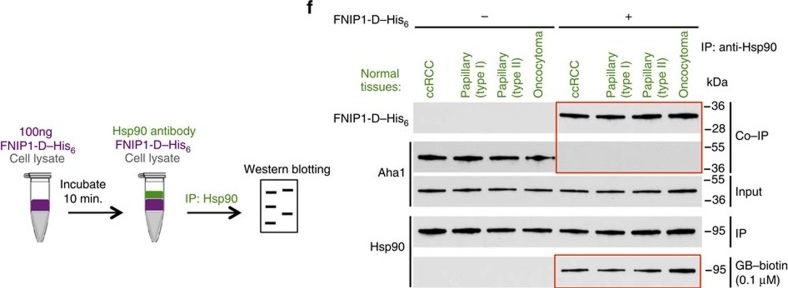
High levels of FNIPs make renal tumours sensitive to Hsp90 inhibitor GB.(a) Clear cell renal cell carcinoma (ccRCC), (b) Papillary type I, (c) Papillary type II, (d) Oncocytoma (Tumours, T) and adjacent normal tissues (Normal, N) were stained with haematoxylin and eosin (H&E). Proteins were also extracted from these tumours and adjacent normal tissues and incubated with indicated amounts of biotinylated GB followed by streptavidin agarose beads. Hsp90 was detected by immunoblotting. Expression of FNIP1 and FNIP2 in these samples was also detected by immunoblotting. (e) Hsp90 immunoprecipitated from tumours (T) and adjacent normal tissues (N) in a–d. Co-IP of FNIPs and Aha1 was examined by immunoblotting. (f) Lysates from normal tissues in a–d were incubated with or without 100 ng of pure FNIP1-D–His6 for 10 min. Hsp90 was immunoprecipitated and co-IP of FNIP1-D–His6 and Aha1 were shown by immunoblotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)
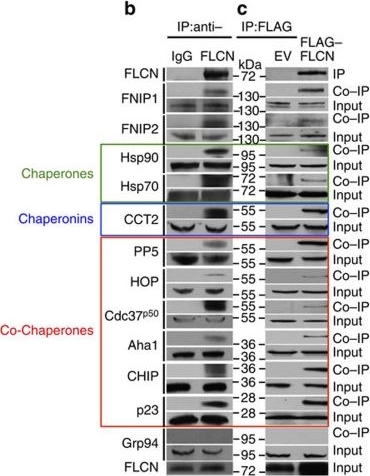
Folliculin is a new client of Hsp90.(a) FLAG–FLCN was expressed and isolated from HEK293 cells. Profile of interacting proteins determined by MALDI–time of flight. Red nodes represent chaperones and co-chaperones, blue nodes are chaperonins and green nodes are splicing factors and ribosomal proteins. (b) FLCN was isolated from HEK293 cell lysates using anti-FLCN or IgG (control) and immunoblotted with indicated antibodies to confirm protein interactions. (c) HEK293 cells were transiently transfected with FLAG–FLCN or empty vector control (EV), immunoprecipitated and immunoblotted with indicated antibodies to confirm interacting proteins. (d) HEK293 cells were treated with 10 μM of the Hsp70 inhibitor JG-98 at the indicated time points. FLCN protein stability in soluble and insoluble fraction was assessed by immunoblotting. (e) HEK293 cells were treated with 1 μM GB at the indicated time points. FLCN protein stability was assessed by immunoblotting. Akt and Phospho-S473-Akt were used as positive controls. (f) Hsp90α–FLAG was transiently expressed in HEK293 cells. Cells were treated with 1 μM GB for the indicated times. Hsp90α–FLAG was immunoprecipitated and co-IP of FLCN was examined by immunoblotting. (g) HEK293 cells were treated with 50 nM of the proteasome inhibitor bortezomib (BZ) for the indicated times. FLCN protein levels were evaluated at the indicated time points by immunoblotting (upper blots). HEK293 cells were also treated with 1 μM GB for 1 h before addition of 50 nM BZ. Immunoblotting was used to evaluate the FLCN level for the indicated time points (lower blots). (h) Empty vector (EV) or FLAG–FLCN was used to transiently transfect HEK293 cells for 24 h then treated for 4 h with either 50 nm BZ or 1 μM GB. FLAG–FLCN was immunoprecipitated and ubiquitination was examined by immunoblotting with a pan-anti-ubiquitin antibody.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun (2016)



















Product Details
| Alternative Name |
Heat shock protein 90 |
|---|---|
| Application |
IHC (PS), IP, WB |
| Application Notes |
Detects a band of ~90kDa by Western blot. |
| Clone |
16F1 |
| Formulation |
Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| Host |
Rat |
| Immunogen |
Native human Hsp90. |
| Isotype |
IgG2a |
| Purity Detail |
Protein G affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from ascites. |
| Species Reactivity |
Beluga, Bovine, Chicken, Dog, Drosophila, Fish, Guinea pig, Hamster, Human, Monkey, Mouse, Mussel, Opossum, Plant, Porcine, Rabbit, Rat, Scallop, Sheep, Xenopus |
| UniProt ID |
P07900 (HSP90alpha), P08238 (HSP90beta) |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Kinesin-1 mediates proper ER folding of the CaV1.2 channel and maintains mouse glucose homeostasis: Tanaka, Y., Farkhondeh, A., et al.; bioRxiv , (2024)
- SUMOylation of protein phosphatase 5 regulates phosphatase activity and substrate release: Sager, R. A., Backe, S. J., et al.; EMBO Rep. 25, 4636 (2024), Abstract
- Kinesin-1 mediates proper ER folding of the CaV1.2 channel and maintains mouse glucose homeostasis: Tanaka, Y., Farkhondeh, A., et al.; EMBO Rep. 25, 4777 (2024), Abstract
- Catalytic inhibitor of Protein Phosphatase 5 activates the extrinsic apoptotic pathway by disrupting complex II in kidney cancer.: Ahanin, E. F., Sager, R. A., et al.; Cell Chem. Biol. 30, 1223 (2023), Reactant(s): Human, Abstract
- Activation of endoplasmic reticulum stress in premature aging via the inner nuclear membrane protein SUN2: Vidak, S., Serebryannyy, L. A., et al.; Cell Rep. 42, 112534 (2023), Abstract
- Methods to Assess the Impact of Hsp90 Chaperone Function on Extracellular Client MMP2 Activity: Votra, S. D., Alsalih, D., et al.; Methods Mol. Biol. 2693, 221 (2023), Abstract
- Therapeutic Effect of Acer tegmentosum Maxim Twig Extract in Bile Duct Ligation-Induced Acute Cholestasis in Mice: U.J. Bae, et al.; J. Med. Food 25, 652 (2022), Abstract
- Improvement in heat stress-induced multiple organ dysfunction and intestinal damage through protection of intestinal goblet cells from prostaglandin E1 analogue misoprostol: H.P. Hii, et al.; Life Sci. 310, 121039 (2022), Abstract
- Tankyrase-mediated ADP-ribosylation is a regulator of TNF-induced death: L. Liu, et al.; Sci. Adv. 8, eabh2332 (2022), Application(s): WB / Reactant(s) Mouse, Abstract
- A specialized Hsp90 co-chaperone network regulates steroid hormone receptor response to ligand: S.J. Backe, et al.; Cell Rep. 40, 111039 (2022), Abstract
- A novel C-terminal heat shock protein 90 inhibitor that overcomes STAT3-Wnt-β-catenin signaling-mediated drug resistance and adverse effects: H.J. Lee, et al.; Theranostics 12, 105 (2022), Abstract
- Primidone blocks RIPK1-driven cell death and inflammation.: Lühder, F., Theilig, F., et al.; Cell Death Differ. 28, 1610 (2021), Application(s): WB / Reactant(s): Mouse, Abstract
- Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice: Caballero, B., Bourdenx, M., et al.; Nat. Commun. 12, 2238 (2021), Abstract
- Activation of endoplasmic reticulum stress via clustering of the inner nuclear membrane protein SUN2: Vidak, S., Serebryannyy, L. A., et al.; bioRxiv , (2021)
- Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome: Bourdenx, M., Martín-Segura, A., et al.; Cell 184, 2696 (2021), Abstract
- Differential Expression of Inflammasome-Related Genes in Induced Pluripotent Stem-Cell-Derived Retinal Pigment Epithelial Cells with or without History of Age-Related Macular Degeneration.: Kaarniranta, K., Hongisto, H., et al.; Int. J. Mol. Sci. 22, (2021), Application(s): WB / Reactant(s): Human, Abstract
- Assessing BRCA1 activity in DNA damage repair using human induced pluripotent stem cells as an approach to assist classification of BRCA1 variants of uncertain significance: M. Ozgencil, et al.; PLoS One 16, e0260852 (2021), Abstract
- Hsp90 chaperone code and the tumor suppressor VHL cooperatively regulate the mitotic checkpoint: M.R. Woodford, et al.; Cell Stress Chaperones 26, 965 (2021), Abstract
- Identification of Niemann-Pick C1 protein as a potential novel SARS-CoV-2 intracellular target: I. García-Dorival, et al.; Antiviral Res. 194, 105167 (2021), Application(s): ELISA, WB / Reactant(s) SARS-CoV-2 coronavirus, Abstract
- Tankyrase-mediated ADP-ribosylation is a novel regulator of TNF-induced death: Liu, L., Sandow, J. J., et al.; bioRxiv , (2021)
- Identification of NPC1 as a novel SARS-CoV-2 intracellular target: Garcia-Dorival, I., Ángel Cuesta-Geijo, M., et al.; bioRxiv , (2020)
- Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90:Client MMP2 Activity and Matrix Proteolysis: Baker-Williams, A. J., Hashmi, F., et al.; Cell Rep. 28, 1894 (2019), Abstract
- MYC paralog-dependent apoptotic priming orchestrates a spectrum of vulnerabilities in small cell lung cancer.: Dammert, M. A., Brägelmann, J., et al.; Nat. Commun. 10, 3485 (2019), Application(s): WB, Abstract
- Hypoxia induced hERG trafficking defect linked to cell cycle arrest in SH-SY5Y cells: D.R. Vaddi, et al.; PLoS One 14, e0215905 (2019), Abstract — Full Text
- Targeted blockade of HSP90 impairs DNA-damage response proteins and increases the sensitivity of ovarian carcinoma cells to PARP inhibition: Gabbasov, R., Benrubi, I. D., et al.; Cancer Biol. Ther. 20, 1035 (2019), Abstract
- Hsp90 middle domain phosphorylation initiates a complex conformational program to recruit the ATPase-stimulating cochaperone Aha1.: Xu, W., Beebe, K., et al.; Nat. Commun. 10, 2574 (2019), Abstract
- Post-translational Regulation of FNIP1 Creates a Rheostat for the Molecular Chaperone Hsp90: R.A. Sager, et al.; Cell Rep. 26, 1344 (2019), Abstract — Full Text
- Mutation of the co-chaperone Tsc1 in bladder cancer diminishes Hsp90 acetylation and reduces drug sensitivity and selectivity.: Bratslavsky, G., Bourboulia, D., et al.; Oncotarget 10, 5824 (2019), Application(s): IP, WB post IP / Reactant(s): Human, Abstract
- Modulation of Protein Quality Control and Proteasome to Autophagy Switch in Immortalized Myoblasts from Duchenne Muscular Dystrophy Patients: M. Wattin, et al.; Int. J. Mol. Sci. 19, E178 (2018), Application(s):Western Blot, Abstract — Full Text
- HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation.: Kijima, T., Prince, T. L., et al.; Sci. Rep. 8, 6976 (2018), Application(s): WB / Reactant(s): Human, Abstract
- Detection and Analysis of Extracellular Hsp90 (eHsp90): S. Cortes, et al.; Methods Mol. Biol. 1709, 321 (2018), Abstract
- Sporadic renal angiomyolipoma in a patient with Birt-Hogg-Dubé: chaperones in pathogenesis: R.A. Sager, et al.; Oncotarget 9, 22220 (2018), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- Selection of stable expressed reference genes in native and vitrified/thawed human ovarian tissue for analysis by qRT-PCR and Western blot: D.A. Nikishin, et al.; J. Assist. Reprod. Genet. 35, 1851 (2018), Application(s): WB / Reactant(s) Human, Abstract
- Systematic Kinase Inhibitor Profiling Identifies CDK9 as a Synthetic Lethal Target in NUT Midline Carcinoma.: Brägelmann, J., Dammert, M. A., et al.; Cell Rep. 20, 2833 (2017), Abstract
- HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation: Kijima, T., Prince, T. L., et al.; bioRxiv , (2017)
- Measurement of Chaperone-Mediated Effects on Polyglutamine Protein Aggregation by the Filter Trap Assay.: van Waarde-Verhagen, M. A. W. H., Kampinga, H. H., et al.; Methods Mol. Biol. 1709, 59 (2017), Abstract
- Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors: R.C. Chai, et al.; Mol. Oncol. 11, 567 (2017), Application(s): WB post IP / Reactant(s) Human, Abstract — Full Text
- The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding.: Bratslavsky, G., Schmidt, L. S., et al.; Nat. Commun. 7, 12037 (2016), Application(s): WB / Reactant(s): Human, Abstract
- Recombinant HSP70 and mild heat shock stimulate growth of aged mesenchymal stem cells: N.V. Andreeva, et al.; Cell Stress Chaperones 21, 727 (2016), Application(s): WB / Reactant(s) Mouse, Abstract — Full Text
- Inverse Relationship between Progesterone Receptor and Myc in Endometrial Cancer: T. Kavlashvili, et al.; PLoS One 11, e0148912 (2016), Reactant(s) Human, Abstract — Full Text
- Cell death is not essential for caspase-1-mediated interleukin-1β activation and secretion: Conos, S. A., Lawlor, K. E., et al.; Cell Death Differ. 23, 1827 (2016), Abstract
- HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1: R. Wang, et al.; Nat. Commun. 7, 10269 (2016), Application(s): Immunoprecipitation, Abstract
- HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death: A.C. Jacobsen, et al.; Cell Death Dis. 14, e2051 (2016), Application(s): Western blots of both mouse and human cell lines, Abstract
- Defective heat shock factor 1 inhibits the growth of fibrosarcoma derived from simian virus 40/T antigen‑transformed MEF cells.: Zhang, Z., Ma, Y., et al.; Mol. Med. Rep. 12, 6517 (2015), Application(s): WB / Reactant(s): Mouse, Abstract
- Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain: M. M. Pearce, et al.; Nat. Commun. 6, 6768 (2015), Application(s): Immunofluorescence / Reactant(s): Human, Abstract
- Identification and quantitative analysis of cellular proteins affected by treatment with withaferin A using A SILAC-based proteomics approach: M. Narayan, et al.; J. Ethnopharmacol. 8741, 30149 (2015), Application(s): Western Blot, Abstract
- Human Genetic Relevance and Potent Antitumor Activity of Heat Shock Protein 90 Inhibition in Canine Lung Adenocarcinoma Cell Lines: F. Clemente-Vicario, et al.; PLoS One 10, e0142007 (2015), Application(s): WB / Reactant(s): Dog, Abstract — Full Text
- Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide.: Cuervo, A. M., Briand, J. P., et al.; Autophagy 11, 472 (2015), Application(s): WB / Reactant(s): Human, Abstract
- The differential regulation of human ACT1 isoforms by Hsp90 in IL-17 signaling: L. Wu, et al.; J. Immunol. 193, 1590 (2014), Abstract
- Berberine induces hERG channel deficiency through trafficking inhibition.: Yang, B., Liu, C., et al.; Cell. Physiol. Biochem. 34, 691 (2014), Application(s): IP, WB / Reactant(s): Human, Abstract
- Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis: Donnelly, B. F., Needham, P. G., et al.; J. Biol. Chem. 288, 13124 (2013), Abstract
- Epigenetic modification restores functional PR expression in endometrial cancer cells.: Xiao, X., Zhang, Y., et al.; Curr. Pharm. Des. 20, 1874 (2013), Reactant(s): Human, Abstract
- Psoriasis-associated variant Act1 D10N with impaired regulation by Hsp90: C. Wang, et al.; Nat. Immunol. 14, 72 (2013), Application(s): WB using MEF and HEK293 extracts, Abstract — Full Text
- Using the heat-shock response to discover anticancer compounds that target protein homeostasis: Santagata, S., Xu, Y. M., et al.; ACS Chem. Biol. 7, 340 (2012), Abstract
- Activation-induced cytidine deaminase-initiated off-target DNA breaks are detected and resolved during S phase: M.G. Hasham, et al.; J. Immunol. 189, 2374 (2012), Application(s): WB using mouse splenic B-cells extracts, Abstract
- Methylglyoxal alters the function and stability of critical components of the protein quality control: C.F. Bento, et al.; PLoS One 5, e13007 (2010), Application(s): WB using human cell lysates, Abstract — Full Text
- Amyloid-β 1-42 induced endocytosis and clusterin/apoJ protein accumulation in cultured human astrocytes: A. Salminen, et al.; Neurochem. Int. 50, 540 (2007), Application(s): WB using human cell lysates, Abstract
- Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis: S.Y. Huang, et al.; Theriogenology 64, 1940 (2005), Application(s): Immunohistochemistry on testis tissue, Abstract
Related Products
HSP90 (human), (native)
ADI-SPP-770
Native human Hsp90, a highly conserved cytosolic chaperone that, in cooperation with co-chaperones, stabilizes client proteins including kinases, transcription factors, and hormone receptors, and is essential for ATP-dependent folding and activation.
| Alternative Name | HSP86, Heat shock protein 90 |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in HeLa cells. |
HSP90α (human), (recombinant)
ADI-SPP-776
Recombinant human Hsp90α, a cytosolic chaperone that cooperates with co-chaperones to stabilize and fold client proteins including kinases, transcription factors, and steroid hormone receptors in an ATP-dependent manner.

| Alternative Name | HSP86, Heat shock protein 90α |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
HSP90β (human), (recombinant)
ADI-SPP-777
Recombinant human Hsp90β, a constitutively expressed cytosolic chaperone that stabilizes and folds key signaling proteins, steroid receptors, and kinases, supporting cell cycle control and stress responses in an ATP-dependent manner.

| Alternative Name | HSP84, Heat shock protein 90β |
|---|---|
| Purity | ≥85% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?