The Hsp70 family of heat shock protiens contains multiple homologs ranging in size from 66-78 kDa, and are the eukaryotic equivalents of the bacterial DnaK. The most studied Hsp70 members include the cytosolic stress-induced Hsp70 (Hsp72), the constitutive cytosolic Hsc70 (Hsp73), and the ER-localized BiP (Grp78). Hsp70 family members contain highly conserved N-terminal ATP-ase and C-terminal protein binding domains. Binding of peptide to Hsp70 is assisted by Hsp40, and stimulates the inherent ATPase activity of Hsp70, facilitating ATP hydrolysis and enhanced peptide binding. Hsp70 nucleotide exchange and substrate binding coordinates the folding of newly synthesized proteins, the re-folding of misfolded or denatured proteins, coordinates trafficking of proteins across cellular membranes, inhibits protein aggregation, and targets the degradation of proteins via the proteasomal pathway.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
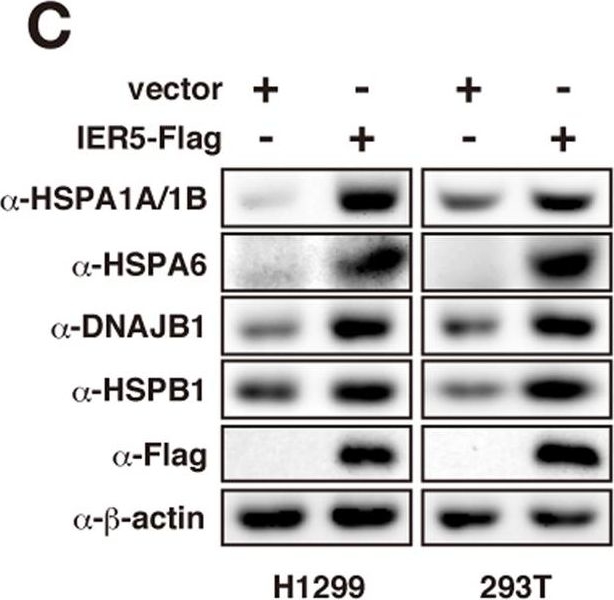
HSP family genes are induced by IER5.(A) H1299 or 293T cells were transfected with control vector or an IER5 expression vector. Cells were harvested 21 hrs or 27 hrs post-transfection and microarray expression analysis was performed. The table shows the HSP family genes, among the genes induced by IER5. (B) H1299 cells were transfected with control, IER5-Flag or mutant IER5-Flag expression vectors (representative image of mut 1 is shown in Fig. S1). Cells were harvested 27 hrs post-transfection, and mRNA expressions of the HSP family genes were analyzed by Northern blotting. (C) H1299 and 293T cells were transfected with control vector or IER5-Flag expression vector, and cells were harvested 24 hrs post-transfection. Expressions of the HSP family proteins were analyzed by Western blotting. (D–F) Control or IER5-targeting siRNAs were introduced into OE33 cells. Cells were harvested 52 hrs post-transfection. Expression of IER5 (D,F) and HSPA1A (E,F) were analyzed by quantitative RT-PCR (D,E) and Western blotting (F). (**p < 0.01). (G) The promoter regions of HSPA1A, HSPA1B and HSPA6 were inserted into the luciferase reporter plasmid containing a minimal promoter, and assayed 24 hrs post-transfection. Experiments were run in triplicate, and data are represented as the mean-fold activation ±SD. (H) Serially deleted regions of the HSPA1A promoter were analyzed as in (G). Numbers indicate the position of the 5′ most nucleotide relative to the transcription initiation site. A heat shock element (HSE), to which HSF1 binds, was found between positions −132 and −109.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: IER5 generates a novel hypo-phosphorylated active form of HSF1 and contributes to tumorigenesis. Sci Rep (2016)
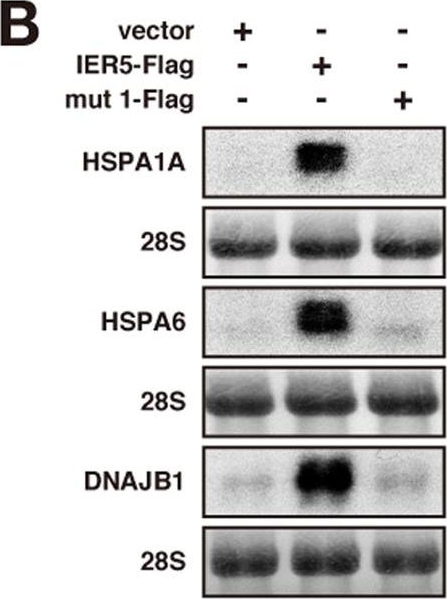
HSP family genes are induced by IER5.(A) H1299 or 293T cells were transfected with control vector or an IER5 expression vector. Cells were harvested 21 hrs or 27 hrs post-transfection and microarray expression analysis was performed. The table shows the HSP family genes, among the genes induced by IER5. (B) H1299 cells were transfected with control, IER5-Flag or mutant IER5-Flag expression vectors (representative image of mut 1 is shown in Fig. S1). Cells were harvested 27 hrs post-transfection, and mRNA expressions of the HSP family genes were analyzed by Northern blotting. (C) H1299 and 293T cells were transfected with control vector or IER5-Flag expression vector, and cells were harvested 24 hrs post-transfection. Expressions of the HSP family proteins were analyzed by Western blotting. (D–F) Control or IER5-targeting siRNAs were introduced into OE33 cells. Cells were harvested 52 hrs post-transfection. Expression of IER5 (D,F) and HSPA1A (E,F) were analyzed by quantitative RT-PCR (D,E) and Western blotting (F). (**p < 0.01). (G) The promoter regions of HSPA1A, HSPA1B and HSPA6 were inserted into the luciferase reporter plasmid containing a minimal promoter, and assayed 24 hrs post-transfection. Experiments were run in triplicate, and data are represented as the mean-fold activation ±SD. (H) Serially deleted regions of the HSPA1A promoter were analyzed as in (G). Numbers indicate the position of the 5′ most nucleotide relative to the transcription initiation site. A heat shock element (HSE), to which HSF1 binds, was found between positions −132 and −109.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: IER5 generates a novel hypo-phosphorylated active form of HSF1 and contributes to tumorigenesis. Sci Rep (2016)


Product Details
| Alternative Name |
Hsp70B prime, Heat shock protein 70 B’ |
|---|---|
| Application |
WB |
| Application Notes |
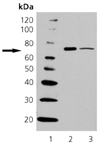
Detects a band of ~70kDa by Western blot. |
| Clone |
165f |
| Formulation |
Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| Host |
Mouse |
| Immunogen |
Synthetic peptide corresponding to the sequence near the C-terminus of human HSP70B’. |
| Isotype |
IgG1 |
| Purity Detail |
Protein G affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, ECL)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from mouse ascites. |
| Species Reactivity |
Human |
| Technical Info / Product Notes |
US Patent No. 7,326,574. |
| UniProt ID |
P17066 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- NGLY1 mutations cause protein aggregation in human neurons.: Manole, A., Wong, T., et al.; Cell Rep. 42, 113466 (2023), Reactant(s): Human, Abstract
- The chaperone DNAJB6 surveils FG-nucleoporins and is required for interphase nuclear pore complex biogenesis: Kuiper, E. F. E., Gallardo, P., et al.; Nat. Cell Biol. 24, 1584 (2022), Abstract
- Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2.: Scieglinska, D., Krawczyk, Z., et al.; Int. J. Mol. Sci. 21, (2020), Reactant(s): Human, Abstract
- The sunless tanning agent dihydroxyacetone induces stress response gene expression and signaling in cultured human keratinocytes and reconstructed epidermis: Perer, J., Jandova, J., et al.; Redox Biol. 36, 101594 (2020), Abstract
- IER5 generates a novel hypo-phosphorylated active form of HSF1 and contributes to tumorigenesis: Y. Asano, et al.; Sci. Rep. 12, 19174 (2016), Application(s): Western blot / Reactant(s): Human, Abstract — Full Text
- Basal and stress-inducible expression of HSPA6 in human keratinocytes is regulated by negative and positive promoter regions.: Aneskievich, B. J., Ramirez, V. P., et al.; Cell Stress Chaperones 20, 95 (2015), Application(s): WB / Reactant(s): Human, Abstract
- TNIP1 reduction of HSPA6 gene expression occurs in promoter regions lacking binding sites for known TNIP1-repressed transcription factors.: Krueger, W., Aneskievich, B. J., et al.; Gene 555, 430 (2015), Application(s): WB / Reactant(s): Human, Abstract
- DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma: C.M. Cabello, et al.; Free Radic. Res. 45, 276 (2011), Abstract
Related Products
| Application | ELISA, IHC, WB |
|---|---|
| Host | Goat |
| Species Reactivity | Mouse |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?