The Hsp70 family of heat shock protiens contains multiple homologs ranging in size from 66-78 kDa, and are the eukaryotic equivalents of the bacterial DnaK. The most studied Hsp70 members include the cytosolic stress-induced Hsp70 (Hsp72), the constitutive cytosolic Hsc70 (Hsp73), and the ER-localized BiP (Grp78). Hsp70 family members contain highly conserved N-terminal ATP-ase and C-terminal protein binding domains. Binding of peptide to Hsp70 is assisted by Hsp40, and stimulates the inherent ATPase activity of Hsp70, facilitating ATP hydrolysis and enhanced peptide binding. Hsp70 nucleotide exchange and substrate binding coordinates the folding of newly synthesized proteins, the re-folding of misfolded or denatured proteins, coordinates trafficking of proteins across cellular membranes, inhibits protein aggregation, and targets the degradation of proteins via the proteasomal pathway.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
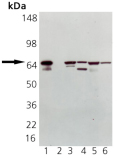
Western blot analysis of HSP70 pAb: Lane 1: HSP70 (HSP72) Recombinant Human Protein, Lane 2: HSC70 (HSP73) Recombinant Bovine Protein (negative control), Lane 3: HeLa Cell Lysate, Heat Shocked, Lane 4: PC-12 Cell Lysate, Heat Shocked, Lane 5: Vero Cell Lysate, Heat Shocked, Lane 6: CHO-K1 Cell Lysate, Heat Shocked
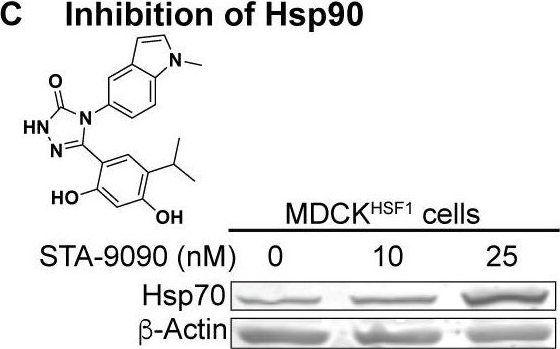
Chemical biology methods to modify the host cell’s proteostasis environment.(A) Destabilized domain technology for stress-independent control of HSF1 activity with trimethoprim (TMP). (B) Dosable induction of HSF1 activity by increasing concentrations of TMP shown by increases in Hsp70 transcripts up to physiologically relevant levels; arsenite is a positive control for endogenous HSF1 activation. Transcript levels normalized to vehicle-treated MDCKYFP cells; error bars represent SEM between biological triplicates. (C) 10 nM STA-9090 does not induce a compensatory heat shock response (representative blot shown; N = 3). Figure 1—figure supplement 1. Validation of chemical biology tools used to perturb proteostasis. Figure 1—figure supplement 2. Heat shock protein transcript expression during influenza infection in modulated proteostasis environments.Validation of chemical biology tools used to perturb proteostasis.(A) Resazurin assay shows that cells treated with small molecules (TMP for cHSF1 and YFP activation; STA-9090 for Hsp90 inhibition) have similar metabolic activity over the course of 72 and 48 hr, respectively, corresponding to the duration of pretreatment and mock-infection. The Y-axis represents the fold-change in fluorescence units relative to vehicle-treated cells; error bars represent SEM between biological triplicates. (B) Chaperone transcript levels in MDCKHSF1 cells (–/+10 μM TMP), relative to vehicle-treated MDCKYFP cells. A typical stress-induced heat shock response is illustrated upon treatment with 100 μM arsenite. The average of biological triplicates is plotted with error bars representing 95% confidence intervals. (C) Chaperone protein levels in HSF1-activated (+10 μM TMP) MDCKHSF1 cells, relative to vehicle-treated MDCKHSF1 cells. The average of biological triplicates is plotted with error bars representing SEM. (D) Cellular thermal shift assay demonstrates STA-9090-mediated Hsp90 stabilization. MDCKHSF1 cells were treated with 0.01% DMSO or 10 nM STA-9090 for 4 hr prior to heating. Error bars represent SEM between three biological replicates.Heat shock protein transcript expression during influenza infection in modulated proteostasis environments.Infections were performed at an MOI of 1 virion/cell; RNA was harvested 8 hr post-infection. 100 μM arsenite was used to induce the heat shock response as a positive control. A transcript encoding the influenza matrix, M, segment was used as a positive control for productive infection; copy number was determined by absolute qPCR. The average of biological triplicates is plotted with error bars representing 95% confidence intervals.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Host proteostasis modulates influenza evolution. Elife (2017)


Product Details
| Alternative Name |
Hsp70, HspA1A, Heat shock protein 70, HspA1B, Hsp72 |
|---|---|
| Application |
IP, WB |
| Application Notes |
Detects a band of ~70kDa by Western blot. |
| Formulation |
Liquid. In PBS containing 50% glycerol and 0.09% sodium azide. |
| GenBank ID |
M11717 |
| Gene/Protein Identifier |
NP_005336.3 (RefSeq), NM_005345 (RefSeq), 3303 (Entrez GeneID), 140550 (OMIM) |
| Host |
Rabbit |
| Immunogen |
Synthetic peptide corresponding to a portion of human Hsp70. |
| Purity Detail |
Protein A affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, ECL)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rabbit serum. |
| Species Reactivity |
Beluga, Bovine, Dog, Hamster, Human, Monkey, Mouse, Porcine, Rat, Sheep |
| UniProt ID |
P0DMV8 (HSPA1A), P0DMV9 (HSPA1B) |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Silica-coated LiYF4:Yb3+, Tm3+ upconverting nanoparticles are non-toxic and activate minor stress responses in mammalian cells: Bietar, K., Chu, S., et al.; RSC Adv. 14, 8695 (2024), Abstract
- Inhibitor Combinations Reveal Wiring of the Proteostasis Network in Prostate Cancer Cells.: Shkedi, A., Adkisson, M., et al.; J. Med. Chem. 64, 14809 (2021), Application(s): WB, Abstract
- Heat shock protein 70 could enhance porcine epidemic diarrhoea virus replication by interacting with membrane proteins.: Shin, H. J., Park, J. E., et al.; Vet. Res. 52, 138 (2021), Reactant(s): Green monkey, Abstract
- Heat acclimation during low-intensity exercise increases V̇O2max and Hsp72, but not markers of mitochondrial biogenesis and oxidative phosphorylation, in skeletal tissue: Mang, Z. A., Fennel, Z. J., et al.; Exp. Physiol. 106, 290 (2021), Abstract
- SUMOylation and the HSF1-Regulated Chaperone Network Converge to Promote Proteostasis in Response to Heat Shock: F. Liebelt, et al.; Cell Rep. 26, 236 (2019), Reactant(s) Human, Abstract — Full Text
- Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization: Hafen, P. S., Abbott, K., et al.; J. Appl. Physiol. 127, 47 (2019), Abstract
- HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis: D. Kennedy, et al.; Cell Death Dis. 8, e3026 (2017), Abstract — Full Text
- Host proteostasis modulates influenza evolution.: Levine, S. S., Butty, V. L., et al.; Elife 6, (2017), Application(s): WB / Reactant(s): Dog, Abstract
- Stabilizing the Hsp70-Tau Complex Promotes Turnover in Models of Tauopathy.: Dickey, C. A., Jinwal, U. K., et al.; Cell Chem. Biol. 23, 992 (2016), Application(s): IP / Reactant(s): Human, Abstract
- Hitting a Moving Target: How Does an N-Methyl Group Impact Biological Activity?: Koay, Y. C., Richardson, N. L., et al.; ChemMedChem 11, 881 (2016), Abstract
- Differential expression patterns among heat-shock protein genes and thermal responses in the whitefly Bemisia tabaci(MEAM 1): F. Diaz, et al.; J. Therm. Biol. 52, 199 (2015), Application(s): Western Blot, Abstract
- Comparative proteomic analysis of the contractile-protein-depleted fraction from normal versus dystrophic skeletal muscle.: Ohlendieck, K., Swandulla, D., et al.; Anal. Biochem. 446, 108 (2014), Reactant(s): Mouse, Abstract
- Application of fluorescence two-dimensional difference in-gel electrophoresis as a proteomic biomarker discovery tool in muscular dystrophy research.: Ohlendieck, K., Swandulla, D., et al.; Biology (Basel) 2, 1438 (2013), Application(s): WB / Reactant(s): Mouse, Abstract
- DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma: C.M. Cabello, et al.; Free Radic. Res. 45, 276 (2011), Abstract
- Downregulation of renal TonEBP in hypokalemic rats: U.S. Jeon, et al.; Am. J. Physiol. Renal Physiol. 293, F408 (2007), Application(s): WB using rat tissue, Abstract
Related Products
HSP70/HSP72 (human), (recombinant)
ADI-NSP-555
A molecular chaperone that assists in the folding of emerging polypeptides and the refolding of denatured proteins.
| Alternative Name | Heat shock protein 70, HspA1A, HspA1B |
|---|---|
| Purity | ≥95% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
HSC70/HSP73 (bovine), (recombinant)
ADI-SPP-751
Recombinant bovine Hsc70/Hsp73, a constitutive cytosolic Hsp70 chaperone that assists protein folding, refolding of misfolded proteins, and proteostasis through ATP-dependent substrate binding and interaction with Hsp40.

| Alternative Name | Heat shock cognate 71 kDa protein, Heat shock 70 kDa protein 8, HSPA8 |
|---|---|
| Purity | ≥90% (SDS-PAGE; Western blot) |
| Source | Produced in E. coli. |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?