Hsp27 is one of the most common members of the highly conserved and ubiquitously expressed family of small heat shock proteins (sHsp), which also includes alphaB-crystallin. It is characterized by a conserved C-terminal alpha-crystallin domain consisting of two anti-parallel beta-sheets that promote oligomer formation required for its primary chaperone function as inhibitor of irreversible protein aggregation. Hsp27 oligomerization is modulated by post-translational phosphorylation of Hsp27 at three serine residues, Ser15, Ser78, and Ser82, by a variety of protein kinases including MAPKAPK-3, PKAc-alpha, p70 S6K, PKD I, and PKC-delta. Hsp27 has been shown to inhibit actin polymerization by binding of unphosphorylated Hsp27 monomers to actin intermediate filaments. Anti-apoptotic functions of Hsp27 have also been identified through interactions with DAXX7, activation of Akt, and inhibition of apoptosome formation. Evidence suggests altered expression of Hsp27 is implicated in the pathogenesis of breast, ovarian, and prostate cancer.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
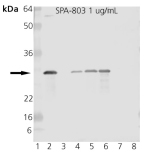
Western blot analysis of HSP27 pAb: Lane 1: MW marker, Lane 2: HSP27 Recombinant Human Protein, Lane 3: HSP25 Recombinant Murine Protein (Negative Control), Lane 4: HeLa, Lane 5: HeLa, Heat Shocked, Lane 6: Vero, Heat Shocked, Lane 7: 3T3, Heat Shocked, Lane 8: PC-12, Heat Shocked.
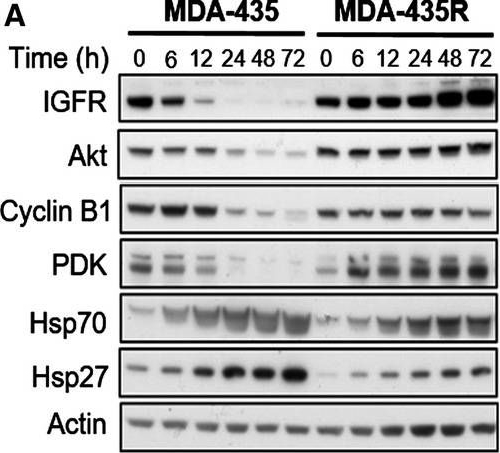
Molecular effects of HSP90 inhibition by 17‐AAG on HSP90 client proteins in parental and resistant cell lines. MDA‐435 parental and resistant MDA‐435R cells treated with 0.1 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (A) and with 30 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (B). MDA‐231 parental and resistant MDA‐231R cells treated with 5.0 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (C) and with 50 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (D). Total cell lysates were collected at the indicated time‐points and analysed by western blotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors. Mol Oncol (2017)
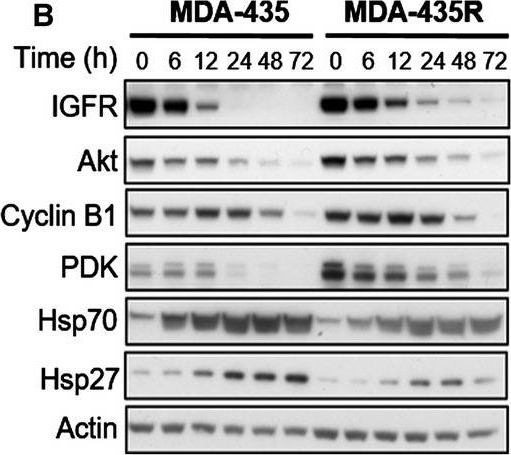
Molecular effects of HSP90 inhibition by 17‐AAG on HSP90 client proteins in parental and resistant cell lines. MDA‐435 parental and resistant MDA‐435R cells treated with 0.1 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (A) and with 30 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (B). MDA‐231 parental and resistant MDA‐231R cells treated with 5.0 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (C) and with 50 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (D). Total cell lysates were collected at the indicated time‐points and analysed by western blotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors. Mol Oncol (2017)
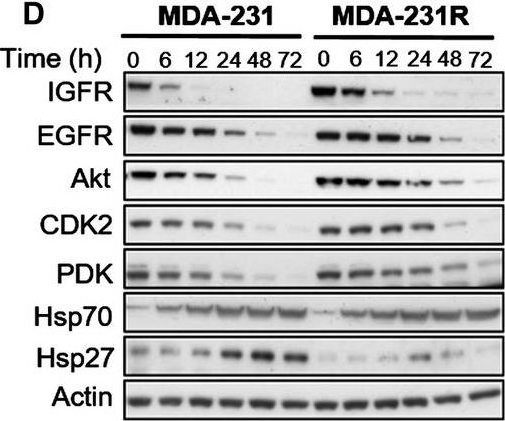
Molecular effects of HSP90 inhibition by 17‐AAG on HSP90 client proteins in parental and resistant cell lines. MDA‐435 parental and resistant MDA‐435R cells treated with 0.1 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (A) and with 30 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (B). MDA‐231 parental and resistant MDA‐231R cells treated with 5.0 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (C) and with 50 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (D). Total cell lysates were collected at the indicated time‐points and analysed by western blotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors. Mol Oncol (2017)
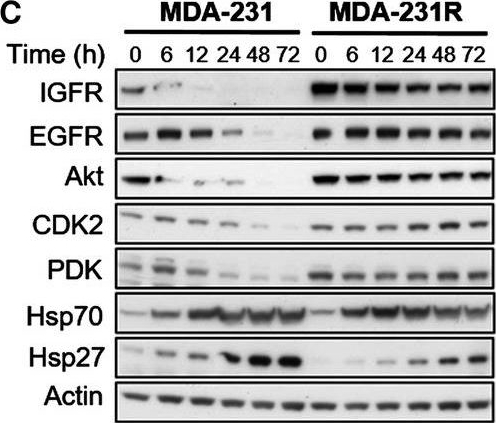
Molecular effects of HSP90 inhibition by 17‐AAG on HSP90 client proteins in parental and resistant cell lines. MDA‐435 parental and resistant MDA‐435R cells treated with 0.1 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (A) and with 30 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (B). MDA‐231 parental and resistant MDA‐231R cells treated with 5.0 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the parental cell line) (C) and with 50 μm of 17‐AAG (~ 5 × 17‐AAG IC50 concentrations of the resistant cell line) (D). Total cell lysates were collected at the indicated time‐points and analysed by western blotting.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors. Mol Oncol (2017)





Product Details
| Alternative Name |
HspB1, Heat shock protein 27 |
|---|---|
| Application |
ELISA, IHC, IP, WB |
| Application Notes |
Detects a band of ~27kDa by Western blot. |
| Formulation |
Liquid. In PBS containing 50% glycerol and 0.09% sodium azide. |
| GenBank ID |
L39370 |
| Host |
Rabbit |
| Immunogen |
Recombinant human Hsp27. |
| Purity Detail |
Protein A affinity purified. |
| Recommendation Dilutions/Conditions |
ELISA (1:1,000)Western Blot (1:1,000, colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rabbit serum. |
| Species Reactivity |
Human, Monkey, Porcine |
| UniProt ID |
P04792 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Overexpression of the human heat shock protein B1 alters obesity-related metabolic changes in a sex-dependent manner in a mouse model of metabolic syndrome.: Ruppert, Z., Sárközy, M., et al.; Biol. Sex Differ. 16, 65 (2025), Abstract
- PXL01 alters macrophage response with no effect on axonal outgrowth or Schwann cell response after nerve repair in rats: Rosberg, D. B. H., Stenberg, L., et al.; Regen. Med. 19, 327 (2024), Abstract
- Reactive astrocytes secrete the chaperone HSPB1 to mediate neuroprotection: Yang, F., Beltran-Lobo, P., et al.; Sci. Adv. 10, eadk9884 (2024), Abstract
- The lysophosphatidic acid-regulated signal transduction network in ovarian cancer cells and its role in actomyosin dynamics, cell migration and entosis.: Ojasalu, K., Lieber, S., et al.; Theranostics 13, 1921 (2023), Application(s): WB / Reactant(s): Human, Abstract
- Resistance to Gemcitabine in Pancreatic Cancer Is Connected to Methylglyoxal Stress and Heat Shock Response: R. Crake, et al.; Cells 12, 1414 (2023), Abstract
- Stress-induced perturbations in intracellular amino acids reprogram mRNA translation in osmoadaptation independently of the ISR: Krokowski, D., Jobava, R., et al.; Cell Rep. 40, 111092 (2022), Abstract
- HSP27/Menin Expression as New Prognostic Serum Biomarkers of Prostate Cancer Aggressiveness Independent of PSA.: Bourefis, A., Berredjem, H., et al.; Cancers (Basel) 14, (2022), Application(s): IHC, Abstract
- Enhanced tumor targeting and timely viral release of mesenchymal stem cells/oncolytic virus complex due to GRP78 and inducible E1B55K expressions greatly increase the antitumor effect of systemic treatment: S. Choi, et al.; Mol. Ther. Oncolytics 27, 26 (2022), Abstract
- Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair.: Kohyama, S., Dahlin, L. B., et al.; Int. J. Mol. Sci. 22, (2021), Reactant(s): Rat, Abstract
- The sunless tanning agent dihydroxyacetone induces stress response gene expression and signaling in cultured human keratinocytes and reconstructed epidermis: Perer, J., Jandova, J., et al.; Redox Biol. 36, 101594 (2020), Abstract
- Methylglyoxal Scavengers Resensitize KRAS-Mutated Colorectal Tumors to Cetuximab: Bellier, J., Nokin, M. J., et al.; Cell Rep. 30, 1400 (2020), Abstract
- A Novel Bead-Based Immunoassay for the Measurement of Heat Shock Proteins 27 and 70.: Verhaeghen, K., Weets, I., et al.; Pathogens 9, (2020), Application(s): WB / Reactant(s): Human, Abstract
- Interval running training improves age-related skeletal muscle wasting and bone loss: Experiments with ovariectomized rats: J.S. Kim, et al.; Exp. Physiol. 104, 691 (2019), Application(s): WB of rat muscle lystates, Abstract
- Retinal pigment epithelium degeneration caused by aggregation of PRPF31 and the role of HSP70 family of proteins.: Massalini, S., Chakarova, C., et al.; Mol. Med. 26, 1 (2019), Application(s): IHC-IF, Abstract
- HSP70 is a negative regulator of NLRP3 inflammasome activation: P. Martine, et al.; Cell Death Dis. 10, 256 (2019), Abstract — Full Text
- Anterior Cingulate Cortex TDP-43 Pathology in Sporadic Amyotrophic Lateral Sclerosis: H. Braak, et al.; J. Neuropathol. Exp. Neurol. 77, 74 (2018), Application(s): IHC, Abstract — Full Text
- Differential expression of heat shock proteins and activation of mitogen-activated protein kinases in A549 alveolar epithelial cells exposed to cigarette smoke extract: Somborac-Bačura, A., Rumora, L., et al.; Exp. Physiol. 103, 1666 (2018), Abstract
- HSP27 is a partner of JAK2-STAT5 and a potential therapeutic target in myelofibrosis: M. Sevin, et al.; Nat. Commun. 9, 1431 (2018), Application(s): Immunoprecipitation, Abstract — Full Text
- SPARC Interacts with Actin in Skeletal Muscle in Vitro and in Vivo: L.H. Jørgensen, et al.; Am. J. Pathol. 187, 457 (2017), Abstract
- Evaluation of geranylgeranylacetone against cisplatin-induced ototoxicity by auditory brainstem response, heat shock proteins and oxidative levels in guinea pigs: W.C. Lo, et al.; Neurotoxicol Teratol. 61, 29 (2017), Abstract
- Differential expression of myocardial heat shock proteins in rats acutely exposed to fluoride: Panneerselvam, L., Raghunath, A., et al.; Cell Stress Chaperones 22, 743 (2017), Abstract
- Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors: R.C. Chai, et al.; Mol. Oncol. 11, 567 (2017), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- Vitamin C and E supplementation does not affect heat shock proteins or endogenous antioxidants in trained skeletal muscles during 12 weeks of strength training.: Cumming, K. T., Raastad, T., et al.; BMC Nutr. 3, 70 (2017), Reactant(s): Human, Abstract
- HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis: D. Kennedy, et al.; Cell Death Dis. 8, e3026 (2017), Abstract — Full Text
- The effect of strength training on muscle cellular stress in prostate cancer patients on ADT.: Ugelstad, I., Raastad, T., et al.; Endocr. Connect. 5, 74 (2016), Application(s): ELISA, WB / Reactant(s): Human, Abstract
- Extracellular HSP110 skews macrophage polarization in colorectal cancer.: Duval, A., Jego, G., et al.; Oncoimmunology 5, e1170264 (2016), Application(s): WB / Reactant(s): Human, Abstract
- Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin: T. Martins-Marques, et al.; J. Extracell. Vesicles 5, 32538 (2016), Application(s): Immunofluorescence staining and histology, tissue samples, Abstract — Full Text
- Dihydropyridine Derivatives Modulate Heat Shock Responses and have a Neuroprotective Effect in a Transgenic Mouse Model of Alzheimer’s Disease: A. Kasza, et al.; J. Alzheimers Dis. 53, 557 (2016), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth: K.H. Su, et al.; Nat. Cell Biol. 18, 527 (2016), Abstract — Full Text
- Ovatodiolide Inhibits Breast Cancer Stem/Progenitor Cells through SMURF2-Mediated Downregulation of Hsp27: K.T. Lu, et al.; Toxins (Basel) 8, E127 (2016), Application(s): Western blot / Reactant(s): Human, Abstract — Full Text
- Expression of HSP27 in Hepatocellular Carcinoma: D. Eto, et al.; Anticancer Res. 36, 3775 (2016), Application(s): Immunohistochemical staining, hepactectomy tumor samples, Abstract — Full Text
- Expression of Heat Shock Proteins (HSPs) in Aged Skeletal Muscles Depends on the Frequency and Duration of Exercise Training.: Lee, Y. H., Yi, H. K., et al.; J. Sports Sci. Med. 14, 347 (2015), Application(s): WB / Reactant(s): Rat, Abstract
- Heat shock protein 27 promotes cell proliferation through activator protein-1 in lung cancer: S. Zhang, et al.; Oncol. Lett. 9, 2572 (2015), Abstract — Full Text
- Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training.: Murton, A. J., Billeter, R., et al.; J. Appl. Physiol. 116, 113 (2014), Reactant(s): Human, Abstract
- Acute response and subcellular movement of HSP27, αB-crystallin and HSP70 in human skeletal muscle after blood-flow-restricted low-load resistance exercise: K.T. Cumming, et al.; Acta Physiol. (Oxf) 211, 634 (2014), Abstract
- Methylglyoxal alters the function and stability of critical components of the protein quality control: C. F. Bento, et al.; PLoS One 5, e13007 (2010), Application(s): WB using human cell lysates, Abstract — Full Text
- The small heat shock protein 27 is a key regulator of CD8+ CD57+ lymphocyte survival: K. Wood, et al.; J. Immunol. 15, 184 (2010), Application(s): EIA using human lymphocytes, Abstract — Full Text
- Chaperone Hsp27, a novel subunit of AUF1 protein complexes, functions in AU-rich element-mediated mRNA decay: K.S. Sinsimer, et al.; Mol. Cell Biol. 28, 5223 (2008), Abstract — Full Text
- Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans: G. Paulsen, et al.; Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R844 (2007), Application(s): EIA using human vastus lateralis muscle tissue, Abstract — Full Text
- Cell death inhibiting RNA (CDIR) derived from a 3′-untranslated region binds AUF1 and heat shock protein 27: L.P. Deiss, et al.; J. Biol. Chem. 277, 47061 (2002), Application(s): WB using human samples, Abstract
- Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity: Y. Tashima, et al.; Nephrol. Dial. Transplant. 14, 1385 (1999), Application(s): WB using porcine samples, Abstract
Related Products

| Application | ELISA, WB |
|---|---|
| Host | Goat |
| Species Reactivity | Rabbit |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?