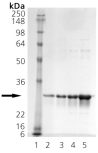
Human Hsp27, mouse Hsp25 and ab-crystallin are part of a diverse family of small heat shock proteins which are produced in all organisms. They function as chaperone-like proteins by binding unfolded polypeptides and preventing uncontrolled protein aggregation. Hsp27 is believed to exist mainly as oligomers of as many as 8-40 Hsp27 protein monomers in cells. Data suggests that the large oligomers of Hsp27 have a chaperone-like activity by serving as a site where unfolded proteins may bind until ATP and Hsp70-dependent refolding can occur. The phosphorylation and oligomerization state of Hsp27 has been suggested to regulate microfilament organization, since only the nonphosphorylated lower molecular weight forms of Hsp27 bind actin barbed ends and inhibit polymerization. Hsp27 is also believed to protect cells by enhancing cellular glutathione levels, as elevated glutathione levels have been measured in cells overexpressing Hsp27. Data from studies using wild-type Hsp27 and mutant forms in which the serine phosphorylation sites were
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: October 9, 2025
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?