The Hsp70 family of heat shock protiens contains multiple homologs ranging in size from 66-78 kDa, and are the eukaryotic equivalents of the bacterial DnaK. The most studied Hsp70 members include the cytosolic stress-induced Hsp70 (Hsp72), the constitutive cytosolic Hsc70 (Hsp73), and the ER-localized BiP (Grp78). Hsp70 family members contain highly conserved N-terminal ATP-ase and C-terminal protein binding domains. Binding of peptide to Hsp70 is assisted by Hsp40, and stimulates the inherent ATPase activity of Hsp70, facilitating ATP hydrolysis and enhanced peptide binding. Hsp70 nucleotide exchange and substrate binding coordinates the folding of newly synthesized proteins, the re-folding of misfolded or denatured proteins, coordinates trafficking of proteins across cellular membranes, inhibits protein aggregation, and targets the degradation of proteins via the proteasomal pathway.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
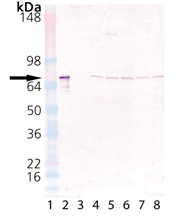
Western Blot Analysis of Hsc70 (Hsp73): Lane 1: MWM, Lane 2: HSC70/HSP70 (bovine), (recombinant) (Cat #: ADI-SPP-751) Lane 3: HSP70/HSP72 (human), (recombinant) (Cat #: ADI-NSP-555), Lane 4: HeLa Cell Lysate, Lane 5: L929 Cell Lysate, Lane 6: PC-12 Cell Lysate, Lane 7: CHO-K1 Cell Lysate, Lane 8: RK-13 Cell Lysate
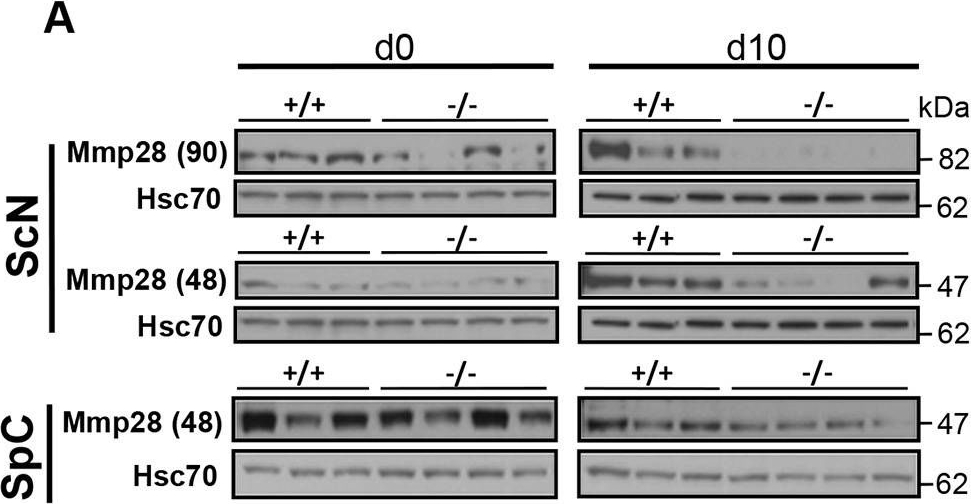
Post-transcriptional dysregulation of Mmp28 in the sciatic nerve. (A) Mmp28 resolves in SDS-PAGE as two distinct electrophoretic isoforms with apparent masses of 48 [Mmp28 (48)] and 90 kDa [Mmp28 (90)]. Immunoblots of equal amounts of homogenates of sciatic nerve (ScN) and spinal cord (SpC) show that Mmp28 (90) and Mmp28 (48) levels are reduced only in −/− sciatic nerve. Hsc70 is a loading control. For ScN d0, the same blots were used for P-Stat3, Stat3, Mmp28 and hsc70 in Figs 7 and 9. For SpC d0, the same blots were used for P-Stat3, Stat3, hnRNPH3, Mmp28 and hsc70 in Figs 7-9. For SpCd10, the same blots were used for P-Stat3, Stat3, Mmp28 and hsc70 in Figs 7 and 9. Lanes shown are experimental replicates. (B) Quantification analyses of immunoblots of Mmp28 in ScN showing a significant decrease in the levels of Mmp28 (90) at d0 and d10, and of Mmp28 (48) at d10 in −/− mice. *P<0.05, Student's t-test, n=4 mice/genotype. Data are expressed as mean±s.d. (C) RT-qPCR showing that the temporal and transcriptional profile of Mmp28 mRNA in the sciatic nerve is not changed between +/+ and −/− mice. Student's t-test, n=4 mice/genotype. Data are expressed as mean±s.d. (D) Representative confocal images of motoneurons in the anterior horn immunostained for Mmp28 show that YFP+ motoneurons do not show changes in the Mmp28 subcellular localization between genotypes. −/−, SLICK-H::Ranbp2flox/flox; +/+, SLICK-H::Ranbp2+/++/+; hsc70, heat shock protein 70; d0 and d10 are days 0 and 10 post-tamoxifen administration, respectively. Scale bars: 25 µm; n.s., non-significant.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis Model Mech (2017)
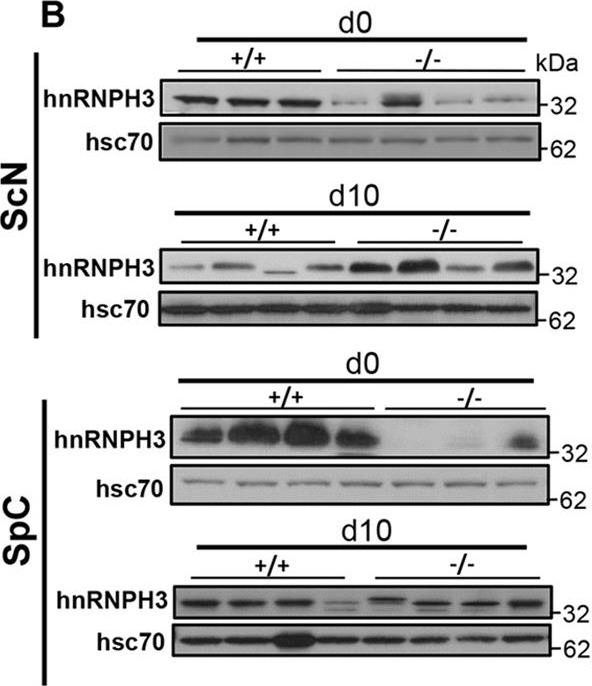
Post-transcriptional deregulation of hnRNPH3 in sciatic nerve and neural cell bodies by loss of Ranbp2. (A) Proteomic analyses by 2D-DIGE of homogenates of optic nerves of +/+ and −/− mice at d10. Insets are volumetric analyses of spot intensities marked by the yellow circle and arrow and that show an increase of a protein, which was identified by mass spectrometry as hnRNPH3, between +/+ and −/− mice. (B) Immunoblots (top panel) of equal amounts of homogenates and quantitative analyses (bottom panel) showing dysregulation of hnRNPH3 proteostasis in sciatic nerve (ScN) and spinal cord (SpC) at d0 and d10. *P<0.05, **P<0.01, Student's t-test, n=4 mice/genotype. Data are expressed as mean±s.d. Hsc70 is a loading control. For SpC d0, the same blots were used for P-Stat3, Stat3, hnRNPH3, Mmp28 and hsc70 in Figs 7-9. A statistical outlier was excluded in the dataset of hnRNPH3 in ScN at d0. Lanes shown are experimental replicates. (C) RT-qPCR showing that the temporal and transcriptional profile of hnRNPH3 mRNA in the sciatic nerve is not changed between +/+ and −/− mice. Student's t-test, n=4 mice/genotype. Data are expressed as mean±s.d. (D,E) Representative confocal images of cross-sections of sciatic nerve (D) and spinal cord (E) immunostained for hnRNPH3. (D) YFP+Hoechst−-axons lack hnRNPH3 and hnRNPH3 is localized in YFP− Hoechst+-Schwann cells, which have enhanced immunostaining of hnRNPH3 in −/− mice at d10. (E) The soma of YFP+ motoneurons in −/− anterior horns conspicuously lack hnRNPH3. Images of a′-d′ and e′-h′ are magnified views of the outlined regions in a-d and e-h, respectively. −/−, SLICK-H::Ranbp2flox/flox; +/+, SLICK-H::Ranbp2+/++/+; hsc70, heat shock protein 70; d0 and d10 are days 0 and 10 post-tamoxifen administration, respectively. Scale bars: 50 µm in Da-p,Ea-h; 20 µm in Ea′-h′; n.s., non-significant.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis Model Mech (2017)
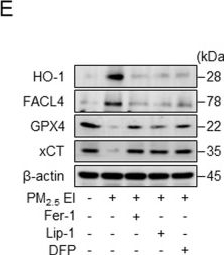
Extraction ion-containing PMs (PM2.5 EI) cause ferroptosis in macrophages.A Western blot analysis after incubation of RAW264.7 cells with three types of PM (100 μg/ml) for 18 h. B Malondialdehyde (MDA) formation in RAW264.7 cells after incubation with 100 μg/ml PMs for 12 h, investigated using a lipid peroxidation assay kit. C Intracellular ferrous iron levels are detected in J774A.1 cells incubated with 50 μg/ml of PMs for 12 h. D WST-8 assay demonstrating the cell viability analysis in RAW264.7 line after pretreatment with ferrostatin-1 (Fer-1; 2 μM), liproxstatin-1 (Lip-1; 2 μM), or deferiprone (DFP; 100 μM) for 2 h followed by the stimulation with 100 μg/ml of PM2.5 EI for 24 h. E Western blot analysis using RAW264.7 cells after preincubation with Fer-1 (2 μM), Lip-1 (2 μM), or DFP (100 μM) for 2 h followed by stimulation with 100 μg/ml of PM2.5 EI for 24 h. F Detection of lipid peroxidation in terms of MDA in J774A.1 cells after preincubation with Fer-1 (2 μM), Lip-1 (2 μM), or DFP (100 μM) for 2 h followed by treatment using 50 μg/ml of PM2.5 EI for 12 h. All data are presented as the means ± standard deviations from at least three independent experiments. *P < 0.05, **P < 0.01 and #P < 0.001. All experiments were conducted at least three times.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages. Cell Death Discov (2024)
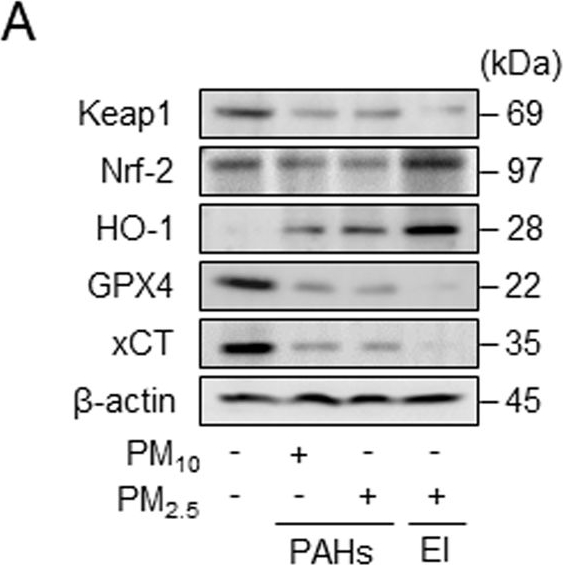
Extraction ion-containing PMs (PM2.5 EI) cause ferroptosis in macrophages.A Western blot analysis after incubation of RAW264.7 cells with three types of PM (100 μg/ml) for 18 h. B Malondialdehyde (MDA) formation in RAW264.7 cells after incubation with 100 μg/ml PMs for 12 h, investigated using a lipid peroxidation assay kit. C Intracellular ferrous iron levels are detected in J774A.1 cells incubated with 50 μg/ml of PMs for 12 h. D WST-8 assay demonstrating the cell viability analysis in RAW264.7 line after pretreatment with ferrostatin-1 (Fer-1; 2 μM), liproxstatin-1 (Lip-1; 2 μM), or deferiprone (DFP; 100 μM) for 2 h followed by the stimulation with 100 μg/ml of PM2.5 EI for 24 h. E Western blot analysis using RAW264.7 cells after preincubation with Fer-1 (2 μM), Lip-1 (2 μM), or DFP (100 μM) for 2 h followed by stimulation with 100 μg/ml of PM2.5 EI for 24 h. F Detection of lipid peroxidation in terms of MDA in J774A.1 cells after preincubation with Fer-1 (2 μM), Lip-1 (2 μM), or DFP (100 μM) for 2 h followed by treatment using 50 μg/ml of PM2.5 EI for 12 h. All data are presented as the means ± standard deviations from at least three independent experiments. *P < 0.05, **P < 0.01 and #P < 0.001. All experiments were conducted at least three times.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages. Cell Death Discov (2024)
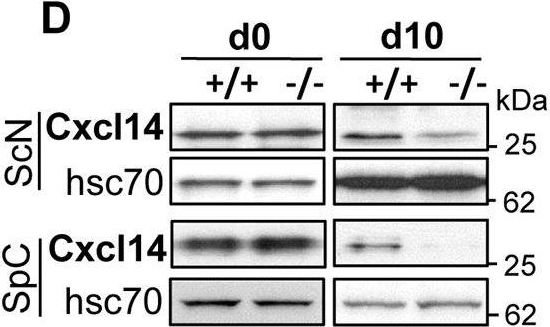
Differential transcriptome and gene expression analyses of sciatic nerve upon loss of Ranbp2 in motoneurons. (A) Differential RNA-Seq-based whole-transcriptome analysis of sciatic nerves between SLICK-H::Ranbp2flox/flox (−/−), SLICK-H::Ranbp2+/+ (+/+) and TgRBD2/3*-HA::SLICK-H::Ranbp2flox/flox (TgRBD2/3*-HA::−/−) mice. Forty-five upregulated and four downregulated transcripts were found in −/− mice at day 10 post-tamoxifen administration. (B) Validation of ranked RNA-Seq dataset by RT-qPCR and temporal and directional changes of expression of mRNAs between −/− and +/+ mice. Twenty-four transcripts and Frzb were confirmed to be uniquely upregulated and downregulated, respectively, by RT-qPCR in −/− sciatic nerve. Cxcl14 had the strongest upregulation (∼10.5-fold) as early as day 3 (d3). *P<0.05, **P<0.01, ***P<0.001, Student's t-test, n=3 or 4 mice/genotype. (C) Temporal and transcriptional changes in expression of Ccl3 in sciatic nerves at d0, d3 and d10. **P<0.01, Student's t-test, n=3 or 4 mice/genotype. (D) Immunoblots (left) and quantification of Cxcl14 (right) from equal amounts of homogenates of sciatic nerves (ScN) and spinal cords (SpC) at d0 and d10. Cxcl14 levels are decreased in ScN and SpC at d10. *P<0.05, Student's t-test, n=4 mice/genotype. Hsc70 is a loading control. All data are expressed as mean±s.d. −/−, SLICK-H::Ranbp2flox/flox; +/+, SLICK-H::Ranbp2+/+; hsc70, heat shock protein 70; d0 and d10 are days 0 and 10 post-tamoxifen administration, respectively.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis Model Mech (2017)
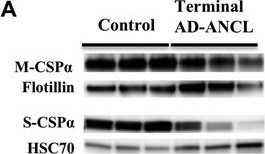
Presynaptic protein levels in the brains of terminal AD-ANCL patients. a Representative western blots (top) and semi-quantitative analysis (graph, bottom) displaying the protein levels of CSPα in the membrane (M-CSPα) (normalized to Flotillin) and cytosolic/soluble (S-CSPα) (normalized to HSC70) fractions from the occipital lobe of three controls and three terminal AD-ANCL patients. The graphs show the expression level of the indicated proteins normalized to flotillin or HSC70 expression. b Representative western blots (top) and semi-quantitative analysis (graph, bottom) show the protein levels of SNAP-25, vesicle-associated membrane protein 2 (VAMP2/ Synaptobrevin), Syntaxin 1 (STX1), and Synaptophysin (SYP) in the occipital lobe from three controls and three terminal AD-ANCL patients in the membrane fraction. Values represent the mean ± S.E.M. of three independent experiments. **, p ≤ 0.01; ***, p ≤ 0.001 using Student’s t test
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Clinically early-stage CSPα mutation carrier exhibits remarkable terminal stage neuronal pathology with minimal evidence of synaptic loss. Acta Neuropathol Commun (2015)







Product Details
| Alternative Name |
Heat shock protein 70, Hsc70, Hsp73 |
|---|---|
| Application |
IHC (PS), WB |
| Application Notes |
Detects a band of ~73kDa by Western blot. |
| Formulation |
Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| GenBank ID |
Y00371 |
| Host |
Rabbit |
| Immunogen |
Synthetic peptide corresponding to a portion of human Hsc70 (Hsp73). The sequence is completely conserved in rat, mouse, hamster and bovine. |
| Purity Detail |
Protein A affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rabbit serum. |
| Species Reactivity |
Bovine, Dog, Guinea pig, Hamster, Human, Monkey, Mouse, Porcine, Rabbit, Rat, Sheep |
| UniProt ID |
P11142 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- MIEN1 promoter deletion leads to impaired migration and invasion potential via actin cytoskeleton rearrangement in colorectal cancer.: Ranade, P., Trivedi, R., et al.; Sci. Rep. 15, 33353 (2025), Abstract
- The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages.: Park, M., Park, S., et al.; Cell Death Discov. 10, 144 (2024), Application(s): WB / Reactant(s): Mouse, Abstract
- Proteostatic remodeling of small heat shock chaperones – crystallins by Ran-binding protein 2 and the peptidyl-prolylcis-transisomerase and chaperone activities of its cyclophilin domain: Patil, H., Cho, K., et al.; bioRxiv , (2024), Reactant(s): Mouse
- Particulate matter induces ferroptosis by accumulating iron and dysregulating the antioxidant system.: Park, M., Cho, Y. L., et al.; BMB Rep. 56, 96 (2023), Reactant(s): Human, Abstract
- Caspase Inhibition Modulates Monocyte-Derived Macrophage Polarization in Damaged Tissues: S. Solier, et al.; Int. J. Mol. Sci. 24, 4151 (2023), Abstract
- Inhibitor Combinations Reveal Wiring of the Proteostasis Network in Prostate Cancer Cells.: Shkedi, A., Adkisson, M., et al.; J. Med. Chem. 64, 14809 (2021), Application(s): WB, Abstract
- Overexpression of Msx1 in Mouse Lung Leads to Loss of Pulmonary Vessels Following Vascular Hypoxic Injury.: West, J., Chen, X., et al.; Cells 10, (2021), Application(s): WB / Reactant(s): Mouse, Abstract
- Osmolytes dynamically regulate mutant Huntingtin aggregation and CREB function in Huntington’s disease cell models: Aravindan, S., Chen, S., et al.; Sci. Rep. 10, 15511 (2020), Abstract
- Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction: Lobanova, E. S., Schuhmann, K., et al.; J. Neurosci. 39, 9689 (2019), Abstract
- Microglial activation in an amyotrophic lateral sclerosis-like model caused by Ranbp2 loss and nucleocytoplasmic transport impairment in retinal ganglion neurons.: Cho, K. I., Yoon, D., et al.; Cell. Mol. Life Sci. 76, 3407 (2019), Reactant(s): Mouse, Abstract
- Heat shock promotes inclusion body formation of mutant huntingtin (mHtt) and alleviates mHtt-induced transcription factor dysfunction: J.Y. Chen, et al.; Jl Biol. Chem. 293, 15581 (2018), Abstract — Full Text
- A new role of anterograde motor Kif5b in facilitating large clathrin-coated vesicle mediated endocytosis via regulating clathrin uncoating: Y.X. Ni, et al.; Cell Discov. 4, 65 (2018), Application(s): ICC-IF, Abstract — Full Text
- Model Based Targeting of IL-6-Induced Inflammatory Responses in Cultured Primary Hepatocytes to Improve Application of the JAK Inhibitor Ruxolitinib: S. Sobotta, et al.; Front. Physiol. 8, 775 (2017), Abstract
- Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes.: Wetsel, W. C., Cho, K. I., et al.; Dis. Model. Mech. 10, 559 (2017), Application(s): WB / Reactant(s): Mouse, Abstract
- Primary fibroblasts from CSPα mutation carriers recapitulate hallmarks of the adult onset neuronal ceroid lipofuscinosis.: Sands, M. S., Benitez, B. A., et al.; Sci. Rep. 7, 6332 (2017), Application(s): WB, Abstract
- Autophagy Activation by Transcription Factor EB (TFEB) in Striatum of HDQ175/Q7 Mice.: Sapp, E., Chase, K., et al.; J. Huntingtons Dis. 5, 249 (2016), Application(s): WB / Reactant(s): Mouse, Abstract
- Deficiency of heat shock transcription factor 1 suppresses heat stress-associated increase in slow soleus muscle mass of mice: Ohno, Y., Egawa, T., et al.; Acta Physiol. (Oxf.) 215, 191 (2015), Abstract
- Clinically early-stage CSPα mutation carrier exhibits remarkable terminal stage neuronal pathology with minimal evidence of synaptic loss.: Cairns, N. J., Morris, J. C., et al.; Acta Neuropathol. Commun. 3, 73 (2015), Application(s): WB / Reactant(s): Human, Abstract
- HSPB8 and the Co-Chaperone BAG3 Are Highly Expressed During the Synthetic Phase of Rat Myometrium Programming During Pregnancy: N.M. Marsh, et al.; Biol. Reprod. 92, 131 (2015), Application(s): Western Blot, Abstract — Full Text
- AICAR-induced activation of AMPK negatively regulates myotube hypertrophy through the HSP72-mediated pathway in C2C12 skeletal muscle cells: T. Egawa, et al.; Am. J. Physiol. Endocrinol. Metab. 306, E344 (2014), Application(s): WB using mouse cell lysate, Abstract — Full Text
- Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity: Chen, Z., Guo, L., et al.; J. Clin. Invest. 124, 3391 (2014), Abstract
- Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis: H.E. Kocalis, et al.; Mol. Metab. 3, 394 (2014), Application(s): Western-blot using mouse tissue, Abstract — Full Text
- Growth differentiation factor-15 deficiency inhibits atherosclerosis progression by regulating interleukin-6-dependent inflammatory response to vascular injury.: Bonaterra, G. A., Zügel, S., et al.; J. Am. Heart Assoc. 1, e002550 (2012), Abstract
- Central nervous system neuropeptide Y signaling via the Y1 receptor partially dissociates feeding behavior from lipoprotein metabolism in lean rats.: Rojas, J. M., Stafford, J. M., et al.; Am. J. Physiol. Endocrinol. Metab. 303, E1479 (2012), Application(s): WB, Abstract
- Rescue of atypical protein kinase C in epithelia by the cytoskeleton and Hsp70 family chaperones: P. Salas, et al.; J. Cell. Sci. 122, 2491 (2009), Application(s): WB using human & mouse cell lysates & tissue, Abstract
- Downregulation of renal TonEBP in hypokalemic rats: U.S. Jeon, et al.; Am. J. Physiol. Renal Physiol. 293, F408 (2007), Application(s): WB using rat tissue, Abstract
- Tid1 Isoforms Are Mitochondrial DnaJ-like Chaperones with Unique Carboxyl Termini That Determine Cytosolic Fate: B. Lu, et al.; J. Biol. Chem. 281, 13150 (2006), Application(s): WB using rabbit samples, Abstract
- Priming biologically active antibody responses against an isolated, conformational viral epitope by DNA vaccination: R. Schirmbeck, et al.; J. Immunol. 169, 1251 (2002), Application(s): WB using human samples, Abstract
- Cancer-associated retinopathy induced by both anti-recoverin and anti-hsc70 antibodies in vivo: I. Maruyama, et al.; Invest. Ophthalmol. Vis. Sci. 40, 3160 (1999), Application(s): IHC, WB using rat samples, Abstract
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
