Hsp70-Hsp90 Organizing Protein (HOP, p60) is an ~60kDa protein that is a critical intermediate component for the efficient maturation of steroid receptor complexes, serving to recruit Hsp90 to Hsp70-containing complexes. HOP contains three tetratricopeptide repeat (TPR) domains, TPR1, TPR2a, and TPR2b.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
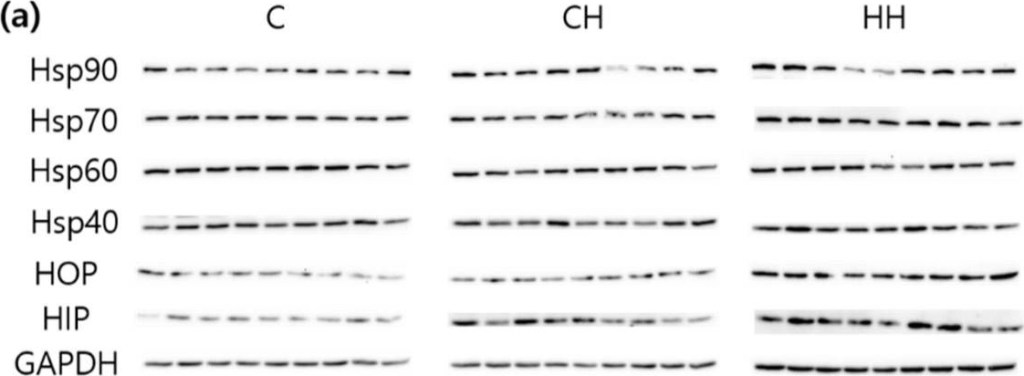
HSPs (heat shock proteins), HOP (hsp70-hsp90 organizing protein), and HIP (hsp70 interacting protein) protein expressions of liver tissue. (a) Bands, each line represents a repetition of each birds; (b) protein expressions level calculated by GAPDH. C, control; CH, chronic heat-stressed broiler; HH, early and chronic heat-stressed broiler. a,b Different superscript letters are significantly different (p < 0.05).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Heat Treatment at an Early Age Has Effects on the Resistance to Chronic Heat Stress on Broilers. Animals (Basel) (2019)
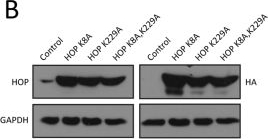
LRET assays of the interactions of TPR mutants of HOP with HSP70 and HSP90. (A) Sequence alignment of the relevant portions of the TPR of CHIP and of TPR1 and TPR2A of HOP; K30 of CHIP, which is known to be important for binding HSP70 and HSP9032,33, is highlighted with a blue arrow. (B) Immunoblot analysis of TPR point mutants; HA-tagged constructs were transiently expressed in HEK293T cells and revealed using both anti-HOP and anti-HA antibodies as indicated with GAPDH as loading control. (C) Co-immunoprecipitation experiments to check the association between HOP mutants and endogenous HSP70 and HSP90; IP, immunoprecipitation; co-IP, coimmunoprecipitation; IB, immunoblot with indicated antibody. The uncropped original images of the immunoblots shown in panels B and C are presented in Supplementary Fig. S2. (D) Luminescence patterns of Tb3+ bound wild-type (LBT-TPR2A WT) and point mutant (LBT-TPR2A K229A). (E) Intrinsic EGFP fluorescence and LRET profiles for wild-type (WT) and point mutant TPR2A. (F) LRET titration experiment comparing the binding of wild-type and mutant TPR2A to HSP90. TPR2A WT and K229A (10 μM) loaded with equimolar Tb3+ were titrated with increasing concentrations of EGFP-C90 (0–6 μM). The Scatchard plot of the normalized LRET from three independent experiments represents means ± SEM. (G) Intrinsic TagRFP fluorescence and LRET profiles for wild-type (WT) and point mutant TPR1. In some panels, the position of the LRET signal is indicated.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Luminescence resonance energy transfer between genetically encoded donor and acceptor for protein-protein interaction studies in the molecular chaperone HSP70/HSP90 complexes. Sci Rep (2018)


Product Details
| Alternative Name |
STIP1, STI1, STUB1, p60, CHIP |
|---|---|
| Application |
IHC, IP, WB |
| Application Notes |
Detects a band of ~60kDa by Western blot. |
| Clone |
DS14F5 |
| Formulation |
Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| Host |
Mouse |
| Immunogen |
Chicken HOP (p60). |
| Isotype |
IgG1 |
| Purity Detail |
Protein G affinity purified. |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000, colorimetric)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from mouse ascites. |
| Species Reactivity |
Bovine, Chicken, Dog, Guinea pig, Hamster, Human, Mink, Monkey, Mouse, Porcine, Rabbit, Rat, Sheep, Xenopus |
| Technical Info / Product Notes |
Recommended by the Human Protein Atlas Organization for IHC (Ensembl No. ENSG00000168439). |
| UniProt ID |
P31948 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Handling |
Avoid freeze/thaw cycles. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Phosphorylation of the Hsp90 Co-Chaperone Hop Changes its Conformational Dynamics and Biological Function: M. Castelli, et al.; J. Mol. Biol. 435, 167931 (2023), Abstract
- Molecular determinants of the crosstalk between endosomal microautophagy and chaperone-mediated autophagy: Krause, G. J., Kirchner, P., et al.; Cell Rep. 42, 113529 (2023), Abstract
- Unraveling the Mechanism of Epichaperome Modulation by Zelavespib: Biochemical Insights on Target Occupancy and Extended Residence Time at the Site of Action.: Sharma, S., Joshi, S., et al.; Biomedicines 11, (2023), Application(s): WB, Abstract
- Experimental traumatic brain injury increases epichaperome formation: S.E. Svirsky, et al.; Neurobiol. Dis. 188, 106331 (2023), Abstract
- Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70: S.Y. Hyun, et al.; Theranostics 11, 2932 (2021), Application(s): Western Blot, Abstract — Full Text
- The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma: M. Heider, et al.; Mol. Cell 81, 1170 (2021), Abstract
- Heat Treatment at an Early Age Has Effects on the Resistance to Chronic Heat Stress on Broilers.: Kang, D., Park, J., et al.; Animals (Basel) 9, (2019), Application(s): WB / Reactant(s): Chicken, Abstract
- Hsp70 and DNAJA2 limit CFTR levels through degradation: Kim Chiaw, P., Hantouche, C., et al.; PLoS One 14, e0220984 (2019), Abstract
- Luminescence resonance energy transfer between genetically encoded donor and acceptor for protein-protein interaction studies in the molecular chaperone HSP70/HSP90 complexes.: Bhattacharya, K., Bernasconi, L., et al.; Sci. Rep. 8, 2801 (2018), Application(s): WB / Reactant(s): Human, Abstract
- A small-molecule Nrf1 and Nrf2 activator mitigates polyglutamine toxicity in spinal and bulbar muscular atrophy: Bott, L. C., Badders, N. M., et al.; Hum. Mol. Genet. 25, 1979 (2016), Abstract
- Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE: K. Saito, et al.; Eur. J. Immunol. 45, 2028 (2015), Abstract — Full Text
- Heat shock protein 70 inhibitors. 2. 2,5′-thiodipyrimidines, 5-(phenylthio)pyrimidines, 2-(pyridin-3-ylthio)pyrimidines, and 3-(phenylthio)pyridines as reversible binders to an allosteric site on heat shock protein 70: Taldone, T., Kang, Y., et al.; J. Med. Chem. 57, 1208 (2014), Abstract
- Psoriasis-associated variant Act1 D10N with impaired regulation by Hsp90: C. Wang, et al.; Nat. Immunol. 14, 72 (2013), Application(s): WB using MEF extracts / Reactant(s): Human, Abstract — Full Text
- A chemical cross-linking method for the analysis of binding partners of heat shock protein-90 in intact cells: Song, S., Kole, S., et al.; Biotechniques , (2012), Abstract
- Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain: S. Tsutsumi, et al.; Nat. Struct. Mol. Biol. 16, 1141 (2009), Application(s): WB, IP using mouse cell lysates, Abstract
- Quantitative assessment of complex formation of nuclear-receptor accessory proteins: A. Jungbauer, et al.; Biochem. J. 345, 627 (2000), Application(s): WB using human samples, Abstract
- Novel oxime derivatives of radicicol induce erythroid differentiation associated with preferential G(1) phase accumulation against chronic myelogenous leukemia cells through destabilization of Bcr-Abl with Hsp90 complex: Y. Shiotsu, et al.; Blood 96, 2284 (2000), Application(s): WB using human samples, Abstract
- Mutation of Hip’s carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly: D.F. Smith, et al.; Mol. Cell. Biol. 18, 944 (1998), Application(s): IP using rabbit samples, Abstract
- The role of DnaJ-like proteins in glucocorticoid receptor.hsp90 heterocomplex assembly by the reconstituted hsp90.p60.hsp70 foldosome complex: W.B. Pratt, et al.; J. Biol. Chem. 273, 7358 (1998), Application(s): IP using rabbit samples, Abstract
- Proteins interacting with the molecular chaperone hsp70/hsc70: physical associations and effects on refolding activity: U. Gehring, et al.; FEBS Lett. 417, 109 (1997), Application(s): WB using human samples, Abstract
Related Products

| Application | ELISA, WB |
|---|---|
| Host | Goat |
| Species Reactivity | Mouse |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?