Homocysteine-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein (HERP) is a membrane-associated ER protein strongly up-regulated by inducers of ER stress.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
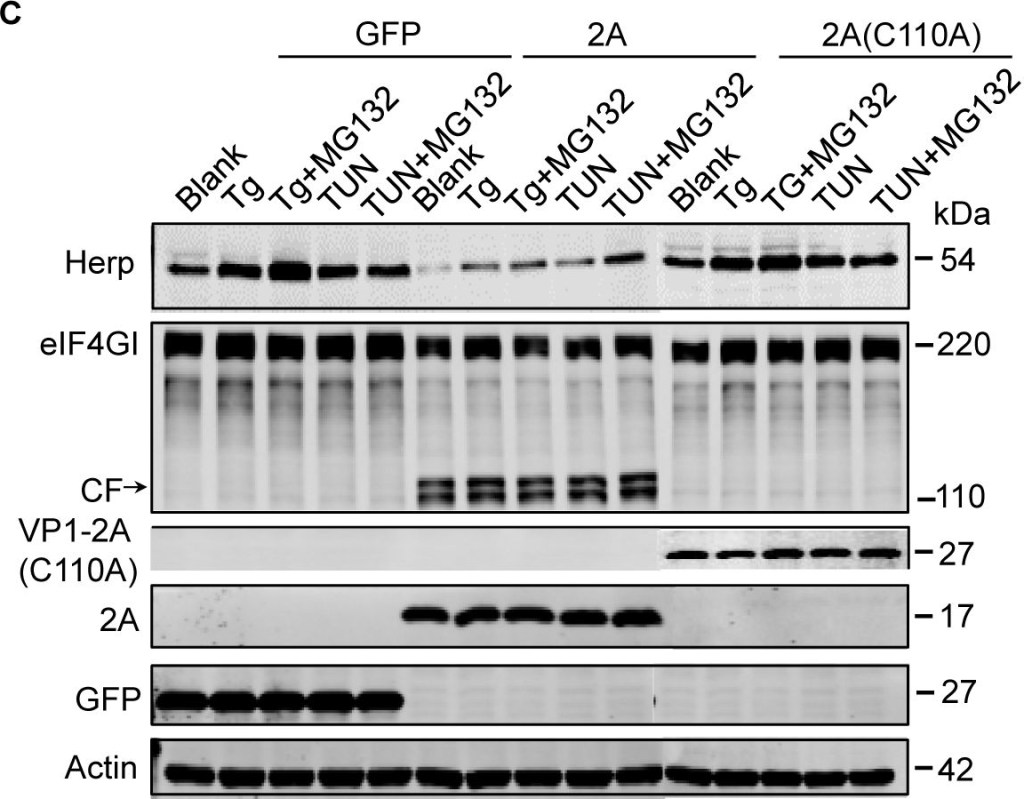
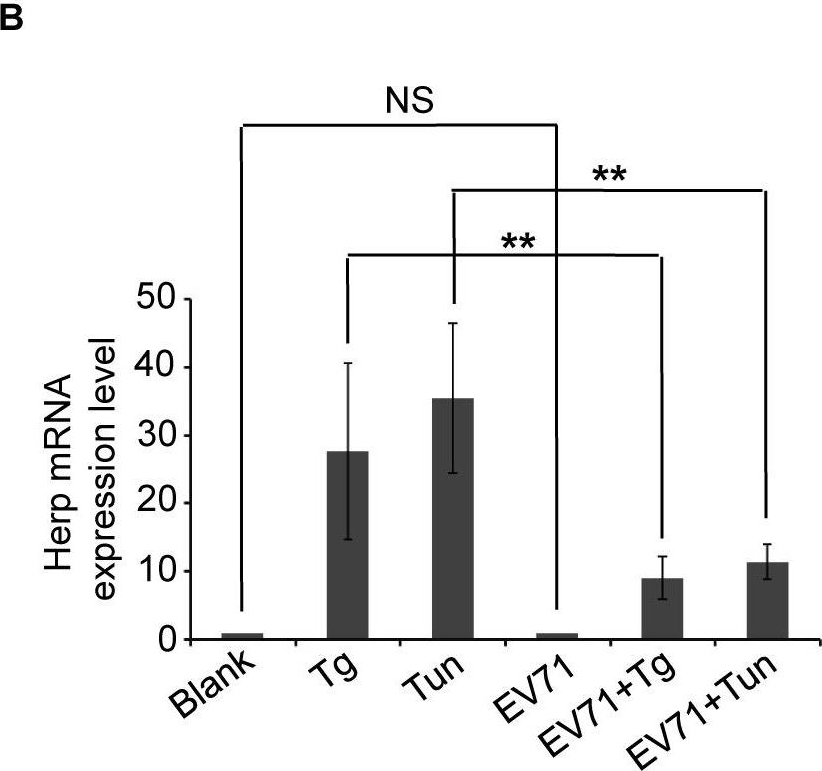
EV71 2Apro inhibits the biosynthesis of Herp and VIMP.(A) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with MG132 (50 μM), Tg (300 nM), Tg plus MG132, Tun (10 μg/ml), Tun plus MG132 for an additional 6 h. Then, the cells were harvested and analyzed by western blotting with antibodies against Herp, EV71 VP1, and actin. The lower panel graph shows the quantification of Herp. The data are presented as means ± SD of two independent experiments. (B) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with Tg (300 nM) or Tun (10 μg/ml) for 6 h to induce Herp expression. Then, the mRNA expression levels of Herp were evaluated by quantitative real-time PCR. The data are presented as means ± SD of two independent experiments. NS, non-significant, P≥0.05; ** P<0.01. (C) BSRT7 cells were transfected with pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A). At 36 h post-transfection, cells were treated with Tg (300 nM), Tg plus MG132 (50 μM), Tun (10 μg/ml), or Tun plus MG132 for 6 h. Then, cell lysates were analyzed by western blotting with antibodies against Herp, eIF4GI, V5, GFP, and actin. eIF4GI was included as a positive control of protease activity of 2Apro, and arrows indicate the cleavage fragments (CF) of eIF4GI. (D) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP plasmid or pcDNA3.1-IRES-2A plasmid (1–3 μg). At 36 h post-transfection, the cells were harvested, RNA was extracted, and quantitative real-time PCR was used to analyze VIMP mRNA expression. The data are presented as means ± SD of three independent experiments. NS, non-significant, P≥0.05. (E) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A) plasmids (1–3 μg). At 36 h post-transfection, the cells were harvested and cell lysates were analyzed by western blotting with antibodies against VIMP, c-MYC, p97, eIF4GI, GFP, V5, and actin.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
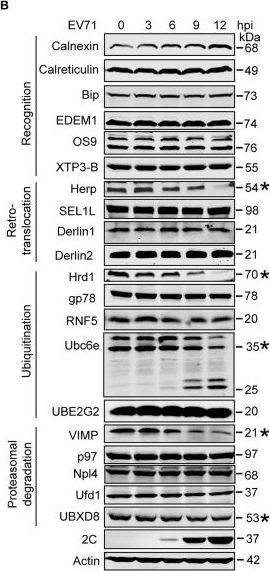
EV71 targets ERAD at multiple points.(A) Diagram of the key molecules involved in ERAD. (B) RD cells were infected with EV71 (MOI = 10) for the indicated times (hpi: hours post-infection). The cells were then harvested and western blotting was performed using the indicated antibodies to detect the indicated ERAD components, EV71 2C, and actin. The ERAD molecules assessed in this study were separated into four categories: substrate recognition, retrotranslocation, ubiquitination, and proteasomal degradation. Asterisks indicate the molecules that were obviously downregulated. (C) Full-size western blots for Herp, Hrd1, VIMP, and UBXD8 described in (B). (D) Quantification of Ubc6e, Herp, Hrd1, VIMP, and UBXD8 in (B). The data are presented as means ± SD of three independent experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
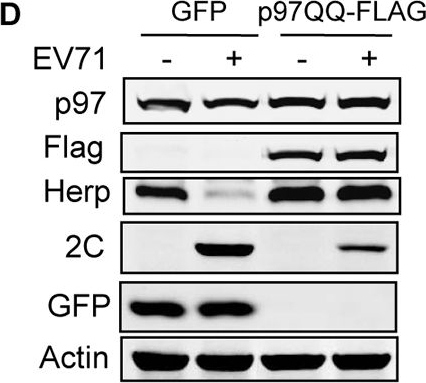
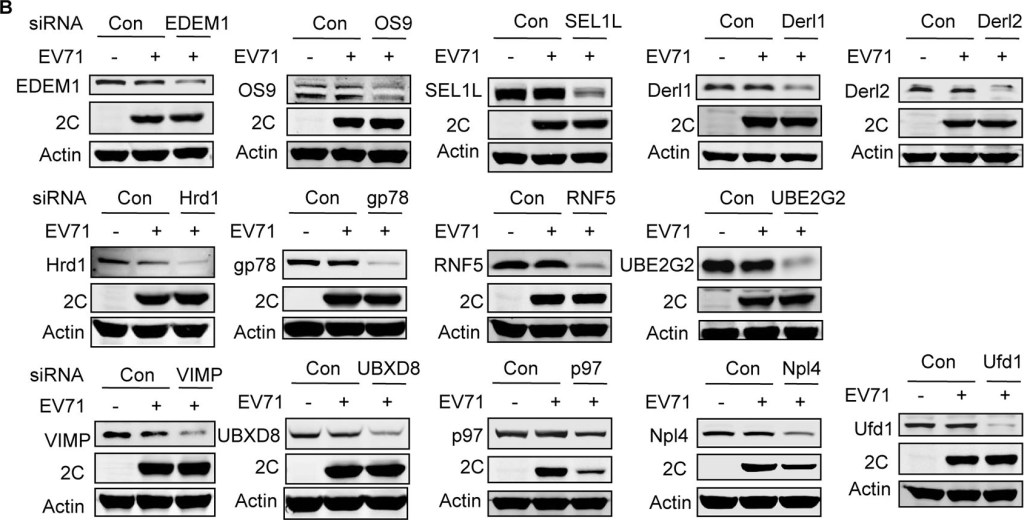
p97 and its ATPase activity are essential for EV71 replication, and it co-localizes with EV71 2C.(A) RD cells were transfected with control siRNA or siRNA targeting Ubc6e or Herp. At 36 h post-transfection, cells were infected with EV71 (MOI = 10) for 12 h. Then real-time PCR was used to qualified viral RNA replication. Data are expressed as fold-change of the EV71 RNA level relative to cells transfected with siRNA control. The data were examined in at least two independent experiments; NS, non-significant, P≥0.05. (B) RD cells were transfected with control siRNA and siRNA targeting different ERAD components including EDEM1, OS9, SEL1L, Derl1, Derl2, Hrd1, gp78, RNF5, UBE2G2, VIMP, UBXD8, p97, Npl4, and Ufd1. At 36 h post-transfection, cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. Then, western blotting was carried out to detect the knockdown efficiency of different molecules, EV71 2C, and actin. (C) RD cells were pre-treated with increasing doses of DBeQ (0–10 μM) for 3 h and then mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. The cell lysates were then analyzed by western blotting with the indicated antibodies. Herp expression was used as an indicator of DBeQ efficiency, and actin was used as the loading control. (D) RD cells were transfected with plasmids encoding GFP or p97QQ-FLAG (dominant negative p97 mutant). At 36 h after transfection, the cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and the cell lysates were analyzed by western blotting with the indicated antibodies. (E) RD cells were mock-infected or infected with EV71 (MOI = 10) for 12 h, and immunostaining was then performed to detect the intracellular distribution of p97 and EV71 2C (p97, green; 2C, red; nuclei, blue). Insets show magnified views of the merged channels in the boxed region. (F) RD cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and cell lysates were immunoprecipitated (IP) with p97 mouse monoclonal antibody or control mouse IgG. Cell lysates and precipitates were analyzed by western blotting with antibodies against p97 and EV71 2C. (G) 293T cells were cotransfected with empty vector (control) or plasmids encoding 2C-HA and p97-FLAG. At 36 h after transfection, cell lysates were immunoprecipitated with HA antibodies. The cell lysates and precipitates were then analyzed by western blotting with antibodies against p97-FLAG and 2C-HA.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
EV71 2Apro inhibits the biosynthesis of Herp and VIMP.(A) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with MG132 (50 μM), Tg (300 nM), Tg plus MG132, Tun (10 μg/ml), Tun plus MG132 for an additional 6 h. Then, the cells were harvested and analyzed by western blotting with antibodies against Herp, EV71 VP1, and actin. The lower panel graph shows the quantification of Herp. The data are presented as means ± SD of two independent experiments. (B) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with Tg (300 nM) or Tun (10 μg/ml) for 6 h to induce Herp expression. Then, the mRNA expression levels of Herp were evaluated by quantitative real-time PCR. The data are presented as means ± SD of two independent experiments. NS, non-significant, P≥0.05; ** P<0.01. (C) BSRT7 cells were transfected with pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A). At 36 h post-transfection, cells were treated with Tg (300 nM), Tg plus MG132 (50 μM), Tun (10 μg/ml), or Tun plus MG132 for 6 h. Then, cell lysates were analyzed by western blotting with antibodies against Herp, eIF4GI, V5, GFP, and actin. eIF4GI was included as a positive control of protease activity of 2Apro, and arrows indicate the cleavage fragments (CF) of eIF4GI. (D) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP plasmid or pcDNA3.1-IRES-2A plasmid (1–3 μg). At 36 h post-transfection, the cells were harvested, RNA was extracted, and quantitative real-time PCR was used to analyze VIMP mRNA expression. The data are presented as means ± SD of three independent experiments. NS, non-significant, P≥0.05. (E) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A) plasmids (1–3 μg). At 36 h post-transfection, the cells were harvested and cell lysates were analyzed by western blotting with antibodies against VIMP, c-MYC, p97, eIF4GI, GFP, V5, and actin.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
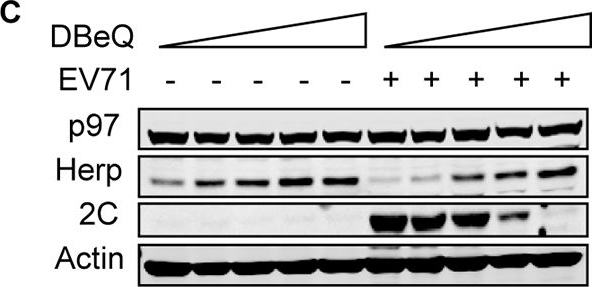
p97 and its ATPase activity are essential for EV71 replication, and it co-localizes with EV71 2C.(A) RD cells were transfected with control siRNA or siRNA targeting Ubc6e or Herp. At 36 h post-transfection, cells were infected with EV71 (MOI = 10) for 12 h. Then real-time PCR was used to qualified viral RNA replication. Data are expressed as fold-change of the EV71 RNA level relative to cells transfected with siRNA control. The data were examined in at least two independent experiments; NS, non-significant, P≥0.05. (B) RD cells were transfected with control siRNA and siRNA targeting different ERAD components including EDEM1, OS9, SEL1L, Derl1, Derl2, Hrd1, gp78, RNF5, UBE2G2, VIMP, UBXD8, p97, Npl4, and Ufd1. At 36 h post-transfection, cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. Then, western blotting was carried out to detect the knockdown efficiency of different molecules, EV71 2C, and actin. (C) RD cells were pre-treated with increasing doses of DBeQ (0–10 μM) for 3 h and then mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. The cell lysates were then analyzed by western blotting with the indicated antibodies. Herp expression was used as an indicator of DBeQ efficiency, and actin was used as the loading control. (D) RD cells were transfected with plasmids encoding GFP or p97QQ-FLAG (dominant negative p97 mutant). At 36 h after transfection, the cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and the cell lysates were analyzed by western blotting with the indicated antibodies. (E) RD cells were mock-infected or infected with EV71 (MOI = 10) for 12 h, and immunostaining was then performed to detect the intracellular distribution of p97 and EV71 2C (p97, green; 2C, red; nuclei, blue). Insets show magnified views of the merged channels in the boxed region. (F) RD cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and cell lysates were immunoprecipitated (IP) with p97 mouse monoclonal antibody or control mouse IgG. Cell lysates and precipitates were analyzed by western blotting with antibodies against p97 and EV71 2C. (G) 293T cells were cotransfected with empty vector (control) or plasmids encoding 2C-HA and p97-FLAG. At 36 h after transfection, cell lysates were immunoprecipitated with HA antibodies. The cell lysates and precipitates were then analyzed by western blotting with antibodies against p97-FLAG and 2C-HA.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
p97 and its ATPase activity are essential for EV71 replication, and it co-localizes with EV71 2C.(A) RD cells were transfected with control siRNA or siRNA targeting Ubc6e or Herp. At 36 h post-transfection, cells were infected with EV71 (MOI = 10) for 12 h. Then real-time PCR was used to qualified viral RNA replication. Data are expressed as fold-change of the EV71 RNA level relative to cells transfected with siRNA control. The data were examined in at least two independent experiments; NS, non-significant, P≥0.05. (B) RD cells were transfected with control siRNA and siRNA targeting different ERAD components including EDEM1, OS9, SEL1L, Derl1, Derl2, Hrd1, gp78, RNF5, UBE2G2, VIMP, UBXD8, p97, Npl4, and Ufd1. At 36 h post-transfection, cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. Then, western blotting was carried out to detect the knockdown efficiency of different molecules, EV71 2C, and actin. (C) RD cells were pre-treated with increasing doses of DBeQ (0–10 μM) for 3 h and then mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h. The cell lysates were then analyzed by western blotting with the indicated antibodies. Herp expression was used as an indicator of DBeQ efficiency, and actin was used as the loading control. (D) RD cells were transfected with plasmids encoding GFP or p97QQ-FLAG (dominant negative p97 mutant). At 36 h after transfection, the cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and the cell lysates were analyzed by western blotting with the indicated antibodies. (E) RD cells were mock-infected or infected with EV71 (MOI = 10) for 12 h, and immunostaining was then performed to detect the intracellular distribution of p97 and EV71 2C (p97, green; 2C, red; nuclei, blue). Insets show magnified views of the merged channels in the boxed region. (F) RD cells were mock infected (−) or infected (+) with EV71 (MOI = 10) for 12 h, and cell lysates were immunoprecipitated (IP) with p97 mouse monoclonal antibody or control mouse IgG. Cell lysates and precipitates were analyzed by western blotting with antibodies against p97 and EV71 2C. (G) 293T cells were cotransfected with empty vector (control) or plasmids encoding 2C-HA and p97-FLAG. At 36 h after transfection, cell lysates were immunoprecipitated with HA antibodies. The cell lysates and precipitates were then analyzed by western blotting with antibodies against p97-FLAG and 2C-HA.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
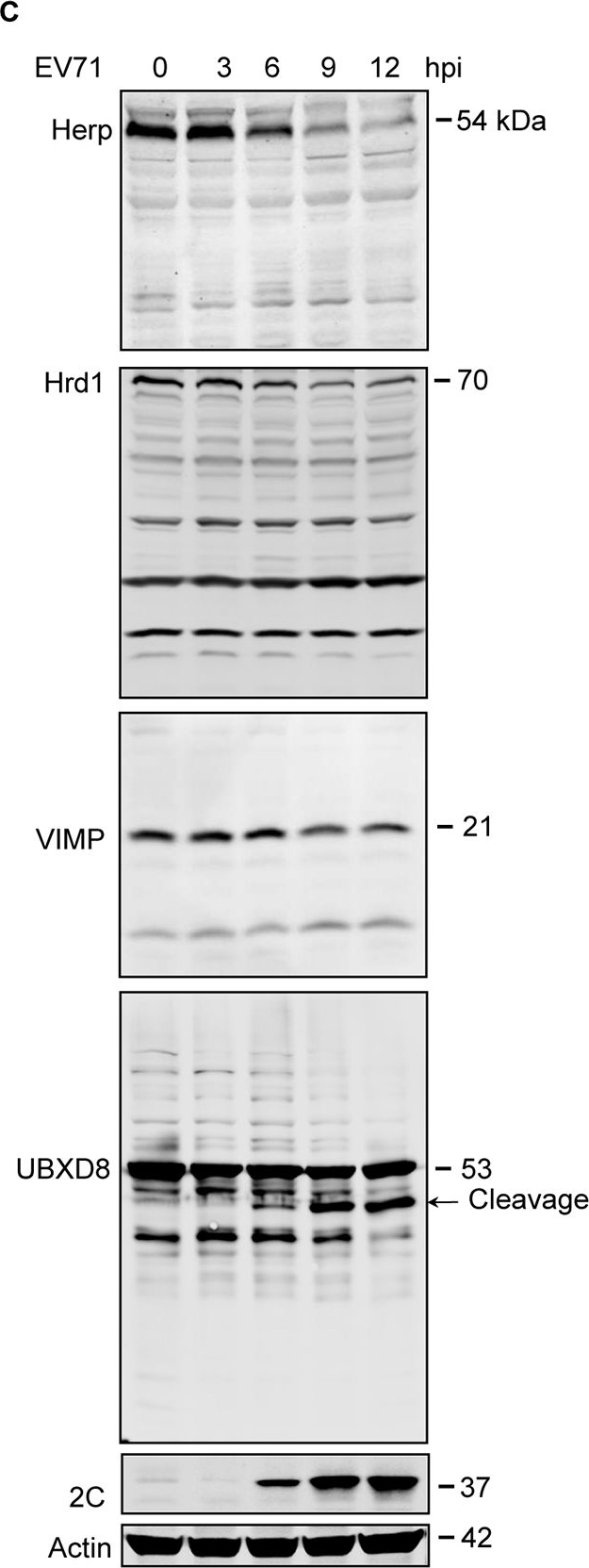
EV71 targets ERAD at multiple points.(A) Diagram of the key molecules involved in ERAD. (B) RD cells were infected with EV71 (MOI = 10) for the indicated times (hpi: hours post-infection). The cells were then harvested and western blotting was performed using the indicated antibodies to detect the indicated ERAD components, EV71 2C, and actin. The ERAD molecules assessed in this study were separated into four categories: substrate recognition, retrotranslocation, ubiquitination, and proteasomal degradation. Asterisks indicate the molecules that were obviously downregulated. (C) Full-size western blots for Herp, Hrd1, VIMP, and UBXD8 described in (B). (D) Quantification of Ubc6e, Herp, Hrd1, VIMP, and UBXD8 in (B). The data are presented as means ± SD of three independent experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)
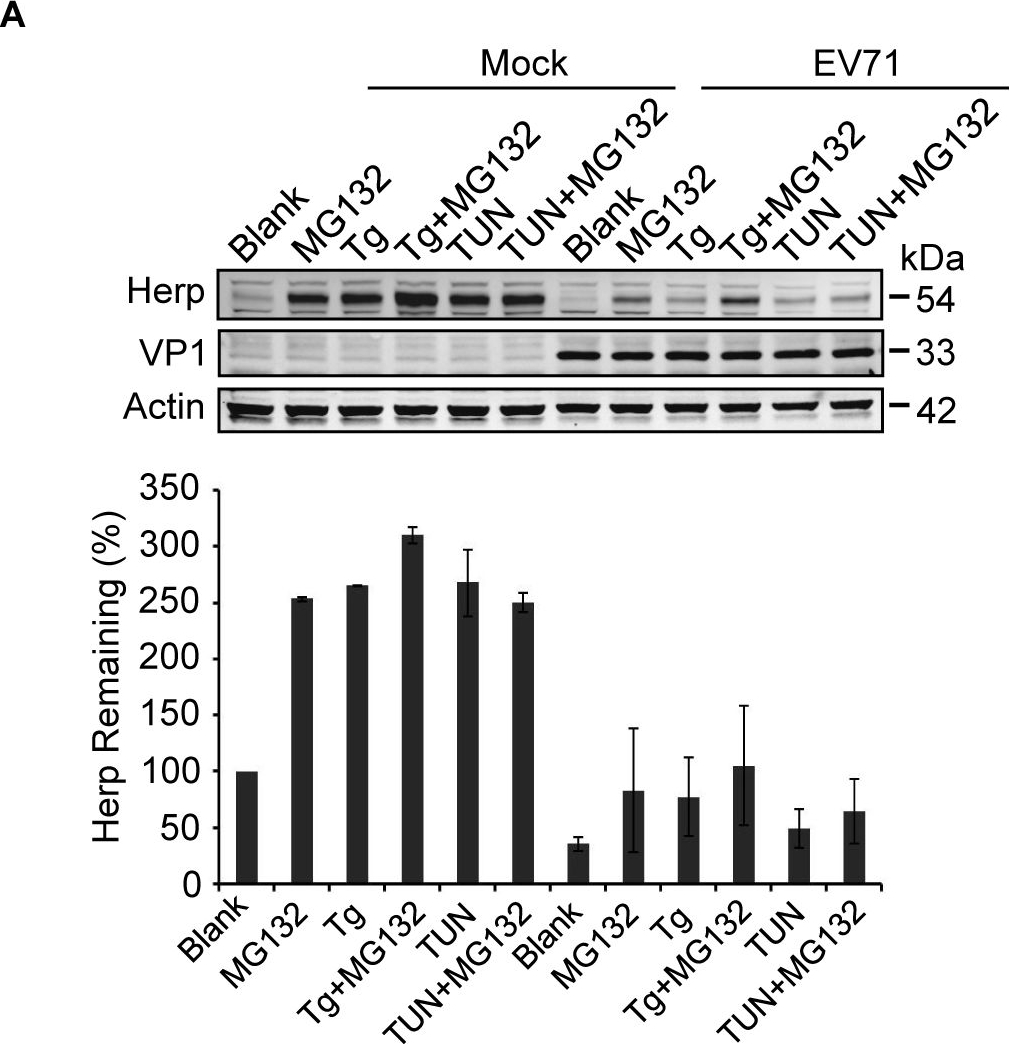
EV71 2Apro inhibits the biosynthesis of Herp and VIMP.(A) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with MG132 (50 μM), Tg (300 nM), Tg plus MG132, Tun (10 μg/ml), Tun plus MG132 for an additional 6 h. Then, the cells were harvested and analyzed by western blotting with antibodies against Herp, EV71 VP1, and actin. The lower panel graph shows the quantification of Herp. The data are presented as means ± SD of two independent experiments. (B) RD cells were mock-infected (−) or infected (+) with EV71 (MOI = 10) for 9 h and then treated with Tg (300 nM) or Tun (10 μg/ml) for 6 h to induce Herp expression. Then, the mRNA expression levels of Herp were evaluated by quantitative real-time PCR. The data are presented as means ± SD of two independent experiments. NS, non-significant, P≥0.05; ** P<0.01. (C) BSRT7 cells were transfected with pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A). At 36 h post-transfection, cells were treated with Tg (300 nM), Tg plus MG132 (50 μM), Tun (10 μg/ml), or Tun plus MG132 for 6 h. Then, cell lysates were analyzed by western blotting with antibodies against Herp, eIF4GI, V5, GFP, and actin. eIF4GI was included as a positive control of protease activity of 2Apro, and arrows indicate the cleavage fragments (CF) of eIF4GI. (D) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP plasmid or pcDNA3.1-IRES-2A plasmid (1–3 μg). At 36 h post-transfection, the cells were harvested, RNA was extracted, and quantitative real-time PCR was used to analyze VIMP mRNA expression. The data are presented as means ± SD of three independent experiments. NS, non-significant, P≥0.05. (E) BSRT7 cells were transfected with increasing doses of pcDNA3.1-EGFP, pcDNA3.1-IRES-2A, or pcDNA3.1-IRES-2A(C110A) plasmids (1–3 μg). At 36 h post-transfection, the cells were harvested and cell lysates were analyzed by western blotting with antibodies against VIMP, c-MYC, p97, eIF4GI, GFP, V5, and actin.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog (2017)








Product Details
| Alternative Name |
Homocystein-responsive endoplasmic reticulum-resident ubiquitin-like domain member 1 protein |
|---|---|
| Application |
IP, WB |
| Formulation |
Liquid. In PBS containing 10mM sodium azide. |
| Host |
Rabbit |
| Immunogen |
Recombinant N-terminal cytoplasmic region of human HERP (aa 1-240). |
| Species Reactivity |
Human |
| UniProt ID |
Q15011 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Long Term Storage |
-20°C |
|---|---|
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- Negative Modulation of Macroautophagy by Stabilized HERPUD1 is Counteracted by an Increased ER-Lysosomal Network With Impact in Drug-Induced Stress Cell Survival.: Tatham, M. H., Hay, R. T., et al.; Front Cell Dev Biol 10, 743287 (2022), Reactant(s): Human, Abstract
- Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication.: Sun, L., Huang, H., et al.; PLoS Pathog. 13, e1006674 (2017), Application(s): WB / Reactant(s): Human, Abstract
- Endoplasmic Reticulum Stress and Autophagy in Homocystinuria Patients with Remethylation Defects: A. Martinez-Pizarro, et al.; PLoS One 11, e0150357 (2016), Application(s): Western blot, Abstract — Full Text
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
