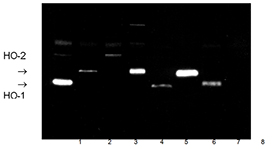
The peptide is totally conserved in pig, rat and mouse HO-1; three amino acid substitutions (A16L; A20G; V24A) exist in the aligned region (residues 31-44) within human, rat and rabbit HO-2; and a single change (A16L) exists in chicken HO.
The rate limiting enzyme in heme catabolism, heme oxygenase, was first described in 1968 and was thought to be a species of cytochrome P450. However, subsequent work showed the enzyme to be a protein distinct from the P450 family and capable of catalysing the degradation of heme to biliverdin and iron, with the concomitant release of carbon monoxide. This enzyme activity is highly conserved throughout the Animal Kingdom and has been identified in algae and plants as well as in all higher species. Heme oxygenase-1 (HO-1) was first purified to homogeneity from rat liver and pig spleen and shown to have a molecular weight of 32kDa. Increased expression of HO-1 can be induced by a large number of structurally unrelated pharmacological and other agents, including cytokines and oxidants, as well as by heat shock and other forms of cellular stress. Baseline levels of HO-1 expression are high in certain tissues, including spleen and liver, but enriched enzyme preparations are recommended for experimental analysis. Recent data have demonstrated a cytoprotective role for HO-1, preventing cell death by modulating intracellular iron levels.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?