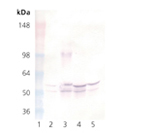
Glycogen Synthase Kinase 3β (GSK-3β) is a unique serine/threonine kinase that is inactivated by phosphorylation. In response to insulin binding, PKB/AKT phosphorylates GSK-3β on serine 9, which prevents GSK-3β from phosphorylating glycogen synthase. Unphosphorylated glycogen synthase is active and able to synthesize glycogen. GSK-3β is also unique in that it requires a substrate that has been phosphorylated by a distinct kinase before it can phosphorylate the substrate. This phosphate priming mechanism explains why phosphorylation of serine 9 inactivates GSK-3β. The phosphorylated serine binds to the GSK-3β priming phosphate position and prevents binding of alternative substrates. In addition to insulin signaling, GSK-3β participates in the Wnt signaling pathway, where it forms a complex with axin, beta-catenin and adenomatous polyposis coli (APC) protein. In the presence of Wnts, GSK-3β is unable to phosphorylate beta-catenin, which leads to stabilization of beta-catenin. The Wnt pathway inactivates GSK-3β via the proteins, Dishevelled and FRAT, which disrupt the interaction of GSK-3β with axin, beta-catenin, and APC. Clinically, there is considerable interest in GSK-3β inhibitors because they may mimic the effect of insulin or reduce the hyperphosphorylation of Tau that is observed in Alzheimer’s Disease.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?