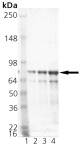
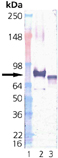
Cells from various organisms increase expression of a class of proteins referred to as heat shock or stress proteins in response to adverse changes in their environment. The members of one class of stress proteins, the Hsp70 family, all bind ATP in vitro, but exist within different intracellular compartments. Members include constitutive form Hsc70 within the cytosol/nucleus; inducible form Hsp70 within the cytosol/nucleus/nucleolus; the constitutive glucose-regulated 78 kDa (or BiP) protein within the lumen of the endoplasmic reticulum; and the constitutive glucose-regulated 75 kDa (Grp75) protein within the mitochondrial matrix. Members of the Hsp70 family appear to function as “molecular chaperones, assisting in the folding of other proteins in various intracellular compartments. Grp75 resides in the mitochondrial matrix where it collaborates with Hsp60 in the re-folding of proteins translocated into this organelle. Like itsE. coli homolog DnaK, Grp75 possesses a cation-dependent ATPase activity considered central to its function as a chaperone.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?