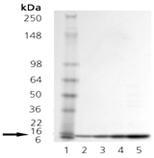
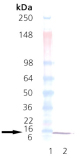
The E. coli heat shock proteins GroEL and GroES are two of the most thoroughly characterized members of a class of proteins known collectively as “molecular chaperones”. GroEL (cpn60; Hsp60 homolog) is an acidic 60kDa E. coli heat shock protein which possesses a weak ATPase activity. In its native form, GroEL exists as a 14-mer consisting of two stacked rings each composed of seven identical subunits of Mr ~57,250. GroES (cpn10; Hsp10 homolog) consists of a single ring of seven identical subunits of Mr ~10,350. Molecular chaperones, such as GroEL and GroES, are thought to function by interacting with transiently exposed interactive surfaces during their nascent synthesis, folding, transport and oligomerization. The net effect of chaperone participation in these processes is a reduction in inappropriate protein-protein interactions that might produce a nonfunctional structure. The GroEL and GroES genes, constituting the GroE operon, are members of the heat shock regulon ofE. coli. Synthesis of the GroE protein, which accounts for approximately 1% of the cellular protein at 37oC, increases to about 10% of the total protein content upon shifting the growth temperature to 46oC.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: October 9, 2025
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?