| Contents |
BML-SE555-9090 SIRT5 (Sirtuin 5) (human, recombinant)
FORM: Dissolved in 25 mM TRIS, pH 7.5, 100 mM NaCl, 5 mM DTT, 1 mg/mL BSA and 10% glycerol.
STORAGE: -70°C; AVOID FREEZE/THAW CYCLES!
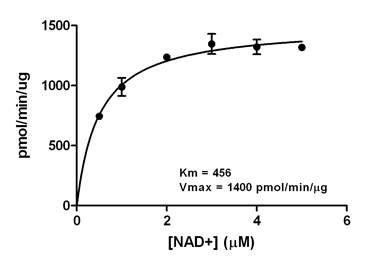
QUANTITY: 1200 U; See vial label for specific activity and protein concentration. One U= 1 pmol/min at 37°C, 250 µM
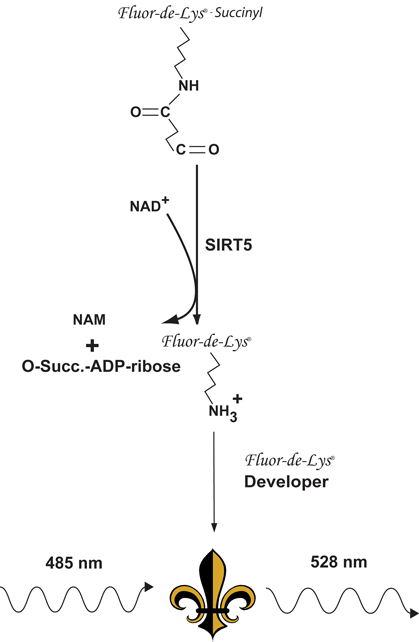
FLUOR DE LYS®–Succinyl Green, Desuccinylase, 2000 µM NAD+.
BML-KI591-0050 FLUOR DE LYS®–Succinyl Green, Desuccinylase Substrate
FORM: 5 mM solution in DMSO (dimethylsulfoxide)
STORAGE: -70°C
QUANTITY: 50 µl
BML-KI105-0300 FLUOR DE LYS®Developer Concentrate (20x)
FORM: 20x Stock Solution; Dilute in Assay Buffer before use.
STORAGE: -70°C
QUANTITY: 300 µl
BML-KI282-0500 NAD+ (Sirtuin Substrate)
FORM: 50 mM b-Nicotinamide adenine dinucleotide (oxidized form) in 50 mM TRIS-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2.
STORAGE: -70°C
BML-KI283-0500 Nicotinamide (Sirtuin Inhibitor)
FORM: 50 mM Nicotinamide in 50 mM TRIS-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2.
STORAGE: -70°C
QUANTITY: 500 µl
BML-KI285-0010 Suramin sodium (Sirtuin Inhibitor)
FORM: Solid
MW: 1429.2
STORAGE: -70°C
QUANTITY: 10 mg
SOLUBILITY: Water or Assay Buffer to 25 mM (10 mg in 0.27 ml)
BML-KI605-0030FLUOR DE LYS®–Green Desuccinylated Standard
FORM: 1 mM in DMSO (dimethylsulfoxide)
STORAGE: -70°C
QUANTITY: 30 µl
BML-KI286-0020 Sirtuin Assay Buffer
(50 mM TRIS-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mg/ml BSA)
STORAGE: -70°C
QUANTITY: 20 ml
80-2409 1/2 VOLUME BLACK NBS MICROPLATE
STORAGE: Room temperature.
|
| Technical Info / Product Notes |
Most sirtuin enzymes, also known as class III histone deactylases (class III HDACs), catalyze a reaction which couples deacetylation of protein Ne-acetyllysine residues to the formation of O-acetyl-ADP-ribose and nicotinamide, from the oxidized form of nicotinamide adenine dinucleotide (NAD+). Some sirtuins, notably human SIRT4 and SIRT6, are reported to catalyze an alternative reaction, the transfer of an ADP-ribosyl group from NAD+ to proteins, although the physiological relevance of these reactions is in question. Sirtuin homologs are found in all forms of life, including the archaea, the bacteria and both unicellular and multicellular eukaryotes. The founding exemplar of the group, Sir2 from baker’s yeast (Saccharomyces cerevisiae), was named for its role in gene-silencing (Silent information regulator 2). Transcriptional silencing by Sir2 is linked to its deacetylation of lysines in the N-terminal tails of the histones in chromatin, hence the classification as a class III HDAC. Lysine deacetylation by sirtuins, however, extends beyond histones. Targets of sirtuin regulatory deacetylation include mammalian transcription factors such as p53, the cytoskeletal protein, tubulin, the bacterial enzyme, acetyl-CoA synthetase and its mammalian homologs.
SIRT5, along with two other mammalian sirtuins, SIRT3 and SIRT4, is localized to the mitochondria. The human SIRT5 gene is located in a chromosomal region in which abnormalities are associated with malignancies, suggesting a possible SIRT5 role in cancer. Thus far, the best studied of SIRT5’s possible physiological roles is the deacetylation, and enhancement of the activity of the mitochondrial matrix enzyme carbamoyl phosphate synthase 1 (CPS1), the rate-limiting enzyme for urea synthesis in the urea cycle. Increased urea synthesis is required for removal of nitrogenous waste (ammonia) during periods of increased amino acid catabolism, including calorie restriction, fasting and the consumption of a high protein diet. Nakagawa et al. report that under these conditions, SIRT5 deacetylation of CPS1 is increased, along with CPS1 activity. At least in the instance of starvation, the increased SIRT5 activity may be attributed to increased levels of the sirtuin co-substrate NAD+ in the mitochondria, which in turn is due to induction of the NAD+ synthetic pathway enzyme nicotinamide phosphoribosyltransferase, (Nampt). It should be noted, however, that two proteomic studies of the mouse mitochondrial “acetylome” are in possible conflict with the CPS1 results of Nakagawa et al. One group observed that calorie restriction increased acetylation at 7 of 24 sites in CPS1, but did not lead to deacetylation at any sites. A comparison of the acetylated proteins of fed and fasted mice found that fasting induced the addition of 4 acetylated sites to CPS1, while only one of five sites present in the fed condition disappeared upon fasting.
An alternative view of SIRT5’s physiological function is that it may primarily involve catalysis of reactions other than deacetylation. SIRT5’s deacetylase activity is detectable but weak with an acetylated histone H4 peptide and with chemically acetylated histones or bovine serum albumin. The catalytic efficiency of SIRT5 with an acetylated histone H3 peptide (kcat/Km = 3.5 s-1 M-1) is orders of magnitude lower than several human and yeast sirtuins (SIRT1, SIRT2, Sir2, Hst2) and more than 20-fold lower than the next weakest deacetylase tested, human SIRT3. Although there is a seeming conflict between the idea of SIRT5 as a non-deacetylase and its effects on CPS1, it should be noted that the rate of SIRT5 deacetylation of CPS1 has not been quantified; the deacetylation was only shown in a qualitative way by western blotting with anti-acetyllysine. Further, although SIRT5 performs an NAD+-dependent activation of CPS1 and an NAD+-dependent deacetylation of CPS1, no mechanistic link between the deacetylation and the activation has been established. The in vitro SIRT5/CPS1 activation experiments were performed with crude mitochondrial matrix lysates, from SIRT5 knockout mice, serving as the CPS1 source. Conceivably, the CPS1 harbored another modification, in addition to acetylation, that SIRT5 reversed in an NAD+-dependent reaction. Consistent with this possibility is recently presented evidence that mitochondrial proteins are lysine-succinylated and that SIRT5 can desuccinylate peptides with efficiencies similar to the deacetylation efficiencies of human SIRTs 1-3.
This latter view of SIRT5’s role is supported by the far greater efficiency SIRT5 displays when assayed with a succinylated FLUOR DE LYS®–Green substrate as opposed to an acetylated one. Use of FLUOR DE LYS®–Succinyl Green (Prod. No. BML-KI591) allows SIRT5 to be assayed at nM concentration, as opposed to the µM concentration typically required for assay with acetyllysine substrates. Quite aside from the savings on enzyme, this is a boon for drug discovery applications. Because a compound IC50 less than half the enzyme concentration cannot be determined, low enzyme concentration is desirable for both screening and subsequent characterization of “hits”.
In addition to its uses in assays of purified SIRT5, the FLUOR DE LYS®–Succinyl Green substrate may be an important discovery tool in the area of oxidative stress responses. Although succinyl-CoA is an abundant metabolite, there is no known lysine succinyltransferase equivalent to the acetyltransferases (HATs). Succinylation of protein lysines does, however, occur via reaction with oxidation products of polyunsaturated fatty acids (PUFAs) and cellular lysine desuccinylase activity apparently extends beyond SIRT5.
|
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?