| Contents |
Nuclear Extract from HeLa Cells (human cervical cancer cell line) (Prod. No. BML-KI140)
(100 µl; In 0.1M potassium chloride, 20mM HEPES/sodium hydroxide, pH 7.9, 20% (v/v) glycerol, 0.2mM ethylenediaminetetraacetic acid, 0.5mM dithiothreitol, 0.5mM PMSF, prepared according to a modification of J.D. Dignam et al. (1983) and S.M. Abmayr et al. (1988)).
Storage: -70°C, avoid freeze/thaw cycles
CHEMILUM DE LYS® Substrate (Prod. No. BML-KI598)
(125 µl; 10mM in DMSO)
Storage: -70°C
CHEMILUM DE LYS® Developer Concentrate (20x) (Prod. No. BML-KI599)
(300 µl; 20x stock solution, dilute in developer buffer before use)
Storage: -70°C
Trichostatin A (HDAC Inhibitor) (Prod. No. BML-GR309-9090) (100 µl; 0.2mM in DMSO)
Storage: -70°C
NAD (Sirtuin Substrate) (Prod. No. BML-KI282)
(500 µl; 50mM β-Nicotinamide adenine dinucleotide (oxidized form) in 50mM TRIS-HCl, pH 8.0, 137mM sodium chloride, 2.7mM potassium chloride, 1mM magnesium chloride)
Storage: -70°C
Nicotinamide (Sirtuin Inhibitor) (Prod. No. BML-KI283)
(500µl; 50mM Nicotinamide in 50mM TRIS-HCl, pH 8.0, 137mM sodium chloride, 2.7mM potassium chloride, 1mM magnesium chloride)
Storage: -70°C
HDAC Assay Buffer (Prod. No. BML-KI143)
(20 ml; 50mM TRIS-HCl, pH 8.0, 137mM sodium chloride, 2.7mM potassium chloride, 1mM magnesium chlroide)
Storage: -70°C
Developer Buffer (Prod. No. BML-KI600) (10ml; 50mM MES, pH 6.0, 40% DMSO)
Storage: -70°C
CHEMILUM DE LYS® Enhancer part A (Prod. No. BML-KI601)
(2 x 1.2ml)
Storage: -70°C
CHEMILUM DE LYS® Enhancer part B (Prod. No. BML-KI602) (0.7ml) Storage: -70°C
1/2 volume white microplate (Prod. No. ADI-80-2406)
Storage: Room temperature
|
| Technical Info / Product Notes |
Histones form the protein core of nucleosomes, the DNA/protein complexes that are the subunits of eukaryotic chromatin. The histones’ N-terminal “tails” are subject to a variety of post-translational modifications, including phosphorylation, methylation, ubiquitination, ADP-ribosylation and acetylation. These modifications have been proposed to constitute a ‘histone code’ with profound regulatory functions in gene transcription. The best studied of these modifications, acetylation of the ε-amino groups of specific histone lysine residues, are catalyzed by histone acetyltransferases (HATs). Histone deacetylases (HDACs) are responsible for hydrolytic removal of these acetyl groups.
Histone hyperacetylation correlates with an open, decondensed chromatin structure and gene activation, while hypoacetylation correlates with chromatin condensation and transcriptional repression. Consistent with this, HATs have been shown to associate with several transcriptional activators and some transcriptional activators have been found to have intrinsic HAT activity. Conversely, HDACs are found to associate with transcriptional repression complexes such as NuRD or those including Sin3.
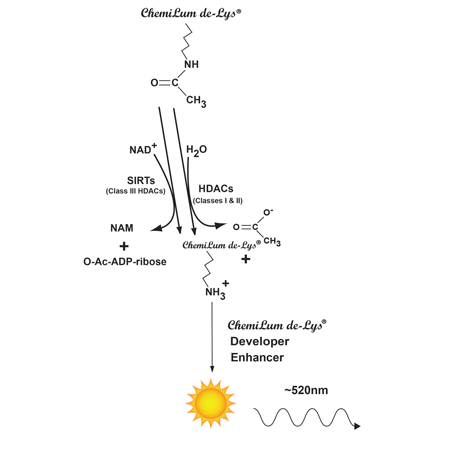
Thus far, eleven human HDACs have been identified, all trichostatin A-sensitive and all homologs of either RPD3 (Class I HDACs) or HDA1 (Class II HDACs), yeast histone deacetylases. Interestingly, Sir2, the yeast mother cell longevity factor, and its mouse homolog, mSir2α, have been shown to be trichostatin A-insensitive, NAD+-dependent histone deacetylases. Human, archaeal and eubacterial Sir2 homologs also display NAD+-dependent histone deacetylase activity. These enzymes apparently function via a unique mechanism, which consumes NAD+ and couples lysine deacetylation to formation of nicotinamide and O-acetyl-ADP-ribose. The Sir2 family (sirtuins) thus constitutes a third class of HDACs, but its members have not been included in the HDAC (Class I/Class II) numbering scheme.
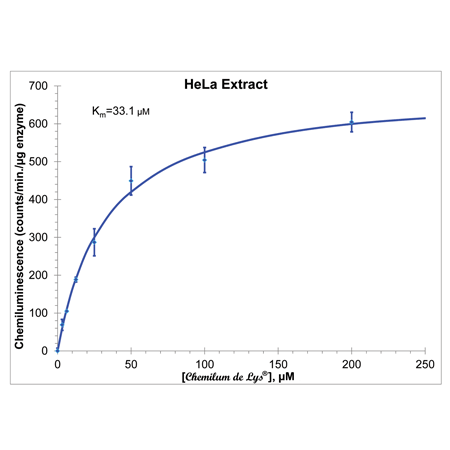
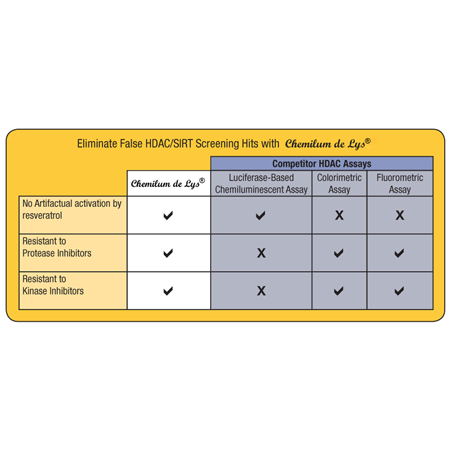
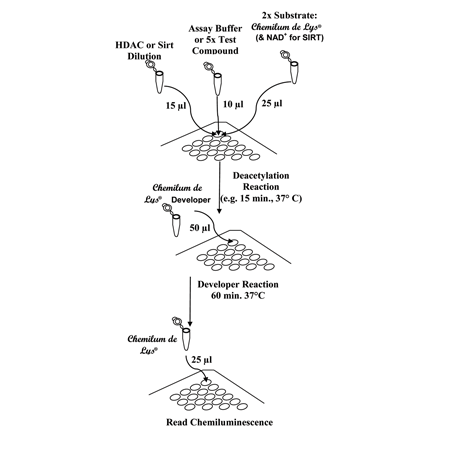
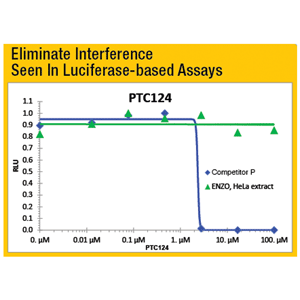
Histone deacetylase inhibitors have shown promise as anti-tumor agents and naturally this has stimulated interest in the screening of compounds for HDAC inhibition. Unfortunately, the standard techniques for HDAC assay are cumbersome. Use of [3H]acetyl-histone or [3H]acetyl-histone peptides as substrates involves an acid/ethyl acetate extraction step prior to scintillation counting. Unlabeled, acetylated histone peptides have been used as substrates, but reactions then require resolution by HPLC. The original FLUOR DE LYS® HDAC assay addressed these problems by providing an assay that can be carried out in two simple mixing steps, all on the same 96-well plate (384-well plates may also be used, but are not included). The CHEMILUM DE LYS® assay has those same advantages, but also, due to chemiluminescent signal, avoids interference by fluorescence from compounds absorbing and/or emitting in the near UV and blue. In addition, the reaction is luciferase-free, thus avoiding compound interference with luciferase activity. The assay has been used successfully with class I, class IIb, class III (sirtuins) and class IV recombinant HDACs. Recent studies have indicated that some compounds shown to activate sirtuins with the fluorescent substrate do not activate deacetylation of the native peptide. The CHEMILUM DE LYS® substrate appears to more closely mimic the natural substrate and does not show this substrate specific activation by resveratrol and other drugs that affect other HDAC or Sirtuin assays.
|
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?