RIG-I (retinoic acid-inducible gene I; Ddx58) and Mda5 (melanoma differentiation-associated gene 5, also known as Ifih1 or Helicard) are proteins that sense viral replication intermediates, such as double-stranded RNA and triggers the host antiviral programs. These molecules signal the downstream activation of NF-κB and IFN regulatory factor (IRF) -3, which coordinately regulate the expression of type-I interferons. Cardif (also called VISA/IPS-1/MAVS) is a CARD (caspase activation and recruitment domain)-containing adaptor protein that interacts with the CARD domain of RIG-I and Mda5, leading to the activation of NF-κB and IRF3. Cardif is located to the mitochondrial outer membrane. Removal of the mitochondrial-targeting domain of cardif abolishes its ability to induce IFNs. Cardif is cleaved and inactivated by NS3-4A, a serine protease from hepatitis C virus known to block interferon-β production.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
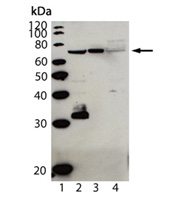
Western blot analysis of Cardif (human), pAb (AT107) (Prod. No. ALX-210-929): Lane 1: MW marker; Lane 2: HepG2; Lane 3: PALA; and Lane 4: HeLa. Additional bands observed probably represent isoforms or cleaved products of Cardif.
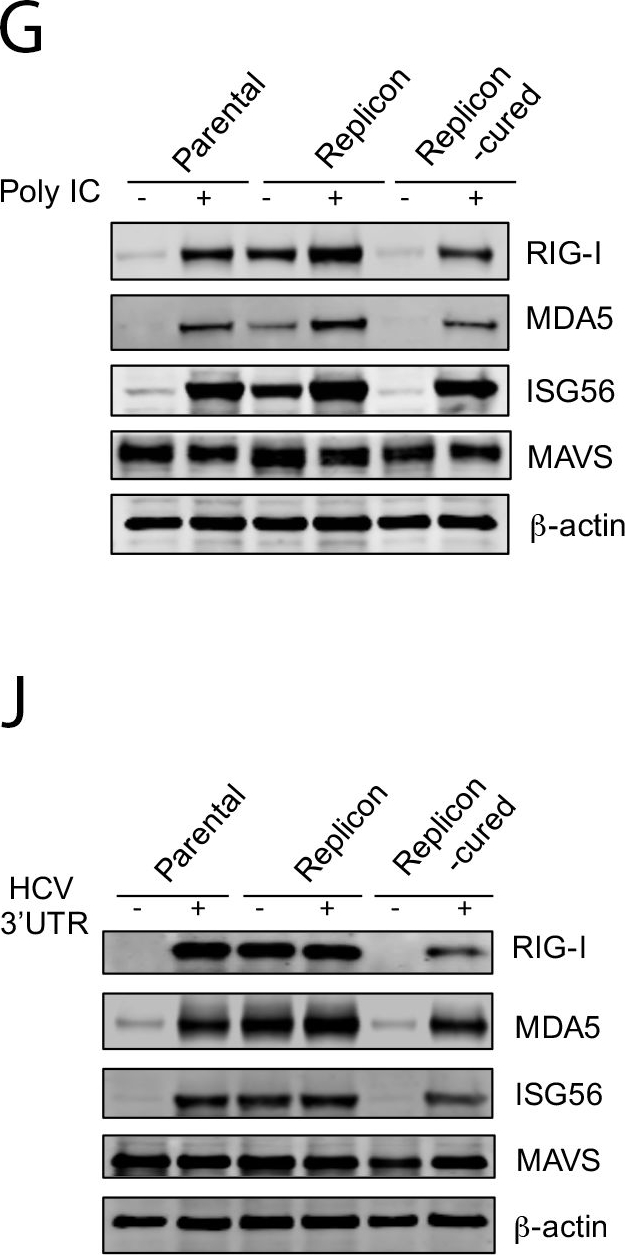
HEV does not target MAVS.(A) Confocal images showing MAVS and viral antigens in HepG2 cells infected with either HEV (top) or HAV (bottom). Cells were stained 5 days after infection with a rabbit anti-MAVS, chimpanzee 1313 serum (HEV), or a murine monoclonal antibody K24F2 (HAV). DAPI was used to stain the nuclei. Scale bar: 10 μm. (B) Confocal images showing the mitochondrial localization of MAVS in HepG2 cells with or without HEV replicon. MAVS was stained with a rabbit antibody against MAVS (green). Mitochondria was visualized with MitoTracker (red). Nuclei were stained with DAPI. Scale bar: 10 μm. (C) HepG2 cells with or without the HEV replicon were transfected with a MAVS-expressing plasmid along with a HAV 3ABC-expressing plasmid or an empty vector. The endogenous (closed arrowheads) and overexpressed MAVS (open arrowheads) were detected with a rabbit anti-MAVS antibody. Note that co-expression of HAV 3ABC led to the degradation of MAVS. (D) HepG2 cells with or without the HEV replicon were transfected with poly IC (6 μg/ml). After 6 h, cells were lysed and subjected to Western blot analysis using antibodies against MAVS and β-actin. Crude mitochondria were isolated and subjected to SDD-AGE for detection of MAVS polymer. (E-F) HepG2 cells with or without the HEV replicon were transfected with poly IC (6 μg/ml). After 6 h, intracellular IFN mRNA levels were measured by qRT-PCR (E) and supernatant IFN-λ concentration was measured by ELISA (F). In panel E, data are expressed as fold changes relative to mock transfected cells containing no replicon, and the results show the mean ± SEM of 2 independent experiments. (G) Immunoblots of endogenous RIG-I, MDA5, ISG56, MAVS, and β-actin in HepG2 cells, replicon cells or replicon-cured cells following poly IC transfection (1.5 μg/ml, 12 h). (H-I) HepG2 cells with or without the HEV replicon were transfected with the hepatitis C virus (HCV) 3’UTR RNA. After 6 h, intracellular IFN mRNA levels were measured by qRT-PCR (H), and concentrations of supernatant IFN-λ were measured by ELISA (I). The results show the mean ± SEM of 2 independent experiments. (J) Immunoblots of endogenous RIG-I, MDA5, ISG56, MAVS, and β-actin in HepG2 cells with or without the replicon before or after HCV 3’UTR RNA transfection (3.6 μg/ml, 14 h).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog (2017)

Signaling pathways involved in HEV-induced IFN response.Immunoblots of RIG-I, MDA5, MAVS and β-actin in HepG2 cells expressing gene-specific shRNA, or GFP (Ctrl). (B-C) IFN-β promoter activity in HepG2 cells with different gene knockdown following Sendai virus (SeV) infection (B) or poly IC transfection (C). Cells were transfected with IFN-β-Luc and TK-RLuc (for normalization of transfection efficiency) 20 h prior to SeV infection or poly IC transfection. Cells were lysed and luciferase activity was determined 20 h after SeV infection or 12 h after poly IC transfection. Data are presented as fold changes relative to non-treated cells. Shown are representative results from two independent experiments each performed in triplicate. (D-F) Effect of RIG-I, MDA5 or MAVS knockdown on HEV replication and host IFN responses. Control and shRNA-expressing HepG2 cells were inoculated with HEV (1000 GE/cell). IFN-λ mRNA expression (D), HEV RNA abundance (E), and HEV-positive foci (F) were determined at 5 days after infection. The results show the mean ± SEM of 2 independent experiments performed in duplicate each. * P<0.05; ** P<0.01. (G) Immunoblots of IRF-3, IRF-7 and β-actin in HepG2 cells transduced with lentiviruses expressing GFP (Ctrl) or gene-specific shRNA. (H-J) Effect of IRF-3 or IRF-7 knockdown on HEV replication and IFN responses. Control and shRNA-expressing cells were inoculated with HEV (1,000 GE/cell). IFN-λ mRNA expression (H), HEV RNA abundance (I), and HEV-positive foci (J) in different cells were determined after 5 days. The results show the mean ± SEM of 2 independent experiments each performed in duplicate wells. * P<0.05; ** P<0.01. Scale bar (upper panel in F), 100 μm.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog (2017)



Product Details
| Alternative Name |
CARD adapter inducing interferon-β, IPS-1, Interferon-β promoter stimulator protein 1, MAVS, Mitochondrial antiviral signalling protein, VISA, Virus-induced signalling adapter |
|---|---|
| Application |
ICC, IP, WB |
| Application Notes |
Detects a band of ~65kDa by Western blot. |
| Formulation |
Liquid. In PBS containing 0.02% sodium azide. |
| Host |
Rabbit |
| Immunogen |
Recombinant human Cardif (CARD adapter inducing interferon-β) (aa 160-450). |
| Purity Detail |
Protein A-affinity purified. |
| Recommendation Dilutions/Conditions |
Immunocytochemistry (1:500)Immunoprecipitation (1:100)Western Blot (1:2,000)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Source |
Purified from rabbit serum. |
| Species Reactivity |
Human |
| UniProt ID |
Q7Z434 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. |
|---|---|
| Handling |
Avoid freeze/thaw cycles. |
| Short Term Storage |
+4°C |
| Long Term Storage |
-20°C |
| Shipping |
Blue Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- The multifaceted role of the viral 2A protease in enterovirus replication and antagonism of host antiviral responses.: Schipper, J. G., Aloise, C., et al.; PLoS Pathog. 21, e1013443 (2025), Abstract
- The critical role of enterovirus 2A protease in viral translation, replication, and antagonism of host antiviral responses: Schipper, J. G., Aloise, C., et al.; bioRxiv , (2025)
- DHX15 and Rig-I Coordinate Apoptosis and Innate Immune Signaling by Antiviral RNase L.: Ramnani, B., Devale, T., et al.; Viruses 16, (2024), Reactant(s): Human, Abstract
- Functional comparisons of the virus sensor RIG-I from humans, the microbatMyotis daubentonii, and the megabatRousettus aegyptiacus, and their response to SARS-CoV-2 infection: Schoen, A., Holzer, M., et al.; bioRxiv , (2023)
- Functional comparisons of the virus sensor RIG-I from humans, the microbat Myotis daubentonii, and the megabat Rousettus aegyptiacus, and their response to SARS-CoV-2 infection.: Schoen, A., Holzer, M., et al.; J. Virol. 97, e0020523 (2023), Application(s): WB / Reactant(s): SARS-CoV-2 coronavirus, Abstract
- High-resolution kinetic characterization of the RIG-I-signaling pathway and the antiviral response.: Burkart, S. S., Schweinoch, D., et al.; Life Sci. Alliance 6, (2023), Reactant(s): Human, Abstract
- Mitochondrial antiviral-signalling protein is a client of the BAG6 protein quality control complex.: High, S., Lawless, C., et al.; J. Cell Sci. 135, (2022), Reactant(s): Human, Abstract
- High Resolution Kinetic Characterization and Dynamic Mathematical Modeling of the RIG-I Signaling Pathway and the Antiviral Responses: Burkart, S. S., Schweinoch, D., et al.; bioRxiv , (2022), Application(s): WB / Reactant(s): Human
- Varicella-Zoster virus ORF9 is an antagonist of the DNA sensor cGAS.: Hertzog, J., Zhou, W., et al.; EMBO J. 41, e109217 (2022), Application(s): WB / Reactant(s): Human, Abstract
- The RNA sensor MDA5 detects SARS-CoV-2 infection: Sampaio, N. G., Chauveau, L., et al.; bioRxiv , (2021)
- MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells: X. Yin, et al.; Cell Rep. 32, 108628 (2021), Application(s): Western Blot, Abstract — Full Text
- The RNA sensor MDA5 detects SARS-CoV-2 infection.: Sampaio, N. G., Bridgeman, A., et al.; Sci. Rep. 11, 13638 (2021), Application(s): WB, Abstract
- Mitochondrial antiviral-signalling protein is a client of the BAG6 protein quality control complex: High, S., Roboti, P., et al.; bioRxiv , (2021), Application(s): IF, IP / Reactant(s): Human
- Varicella-Zoster Virus ORF9 Is an Antagonist of the DNA Sensor cGAS: Hertzog, J., Rigby, R. E., et al.; bioRxiv , (2020)
- RNase L amplifies Interferon signaling by inducing PKR-mediated antiviral stress granules: Malathi, K., Siddiqui, M. A., et al.; bioRxiv , (2020), Reactant(s): Human
- RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules: Manivannan, P., Siddiqui, M. A., et al.; J. Virol. 94, (2020), Abstract
- RIG-I-like receptor activation drives type I IFN and antiviral signaling to limit Hantaan orthohantavirus replication.: Kell, A. M., Hemann, E. A., et al.; PLoS Pathog. 16, e1008483 (2020), Application(s): WB, Abstract
- Dissecting distinct proteolytic activities of FMDV Lpro implicates cleavage and degradation of RLR signaling proteins, not its deISGylase/DUB activity, in type I interferon suppression.: de Los Santos, T., Medina, G. N., et al.; PLoS Pathog. 16, e1008702 (2020), Application(s): WB, Abstract
- PA-X antagonises MAVS-dependent accumulation of early type I interferon messenger RNAs during influenza A virus infection: Rigby, R. E., Wise, H. M., et al.; Sci. Rep. 9, 7216 (2019), Abstract
- Comparative Analysis of African and Asian Lineage-Derived Zika Virus Strains Reveals Differences in Activation of and Sensitivity to Antiviral Innate Immunity: Esser-Nobis, K., Aarreberg, L. D., et al.; J. Virol. 93, (2019), Abstract
- Basal expression of interferon regulatory factor 1 drives intrinsic hepatocyte resistance to multiple RNA viruses: Yamane, D., Feng, H., et al.; Nat. Microbiol. 4, 1096 (2019), Abstract
- DHX15 Is a Coreceptor for RLR Signaling That Promotes Antiviral Defense Against RNA Virus Infection: S. Pattabhi, et al.; J. Interferon Cytokine Res. 39, 331 (2019), Abstract
- Interleukin-1β Induces mtDNA Release to Activate Innate Immune Signaling via cGAS-STING: Aarreberg, L. D., Esser-Nobis, K., et al.; Mol. Cell 74, 801 (2019), Abstract
- The 14-3-3η chaperone protein promotes antiviral innate immunity via facilitating MDA5 oligomerization and intracellular redistribution: J.P. Lin, et al.; PLoS Pathog. 15, e1007582 (2019), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- Mitochondrial double-stranded RNA triggers antiviral signalling in humans: Dhir, A., Dhir, S., et al.; Nature 560, 238 (2018), Abstract
- Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling: J. Hertzog, et al.; Eur. J. Immunol. 48, 1120 (2018), Application(s): WB, Abstract — Full Text
- Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy: G.L. McLelland, et al.; Elife 7, e32866 (2018), Abstract — Full Text
- Palmitoylation mediates membrane association of hepatitis E virus ORF3 protein and is required for infectious particle secretion: J. Gouttenoire, et al.; PLoS Pathog. 14, e1007471 (2018), Abstract
- NSs Protein of Sandfly Fever Sicilian Phlebovirus Counteracts Interferon (IFN) Induction by Masking the DNA-Binding Domain of IFN Regulatory Factor 3: J.D. Wuerth, et al.; J. Virol. 92, e01202-18 (2018), Application(s): WB, Abstract — Full Text
- Disruption of MDA5-Mediated Innate Immune Responses by the 3C Proteins of Coxsackievirus A16, Coxsackievirus A6, and Enterovirus D68: Y. Rui, et al.; J. Virol. 91, e00546-17 (2017), Abstract — Full Text
- DDX3 directly regulates TRAF3 ubiquitination and acts as a scaffold to coordinate assembly of signalling complexes downstream of MAVS: L. Gu, et al.; Biochem. J. 474, 571 (2017), Abstract
- NLRX1 promotes immediate IRF1-directed antiviral responses by limiting dsRNA-activated translational inhibition mediated by PKR: H. Feng, et al.; Nat. Immunol. 18, 1299 (2017), Abstract — Full Text
- Hepatitis E virus persists in the presence of a type III interferon response.: Feng, Z., Yin, X., et al.; PLoS Pathog. 13, e1006417 (2017), Application(s): WB / Reactant(s): Human, Abstract
- A phosphomimetic-based mechanism of dengue virus to antagonize innate immunity: Chan, Y. K., Gack, M. U., et al.; Nat. Immunol. 17, 523 (2016), Abstract
- Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system: P. Bellecave, et al.; Hepatology 51, 1127 (2010), Abstract
- Cleavage of the IPS-1/Cardif/MAVS/VISA does not inhibit T cell-mediated elimination of hepatitis C virus non-structural 3/4A-expressing hepatocytes: G. Ahlen, et al.; Gut 58, 560 (2009), Abstract
- Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity: Y.M. Loo, et al.; J. Virol. 82, 335 (2008), Abstract
- Antiviral suppression vs restoration of RIG-I signaling by hepatitis C protease and polymerase inhibitors: Y. Liang, et al.; Gastroenterology 135, 1710 (2008), Abstract
- The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases: M. Rebsamen, et al.; Cell Death Differ. 15, 1804 (2008), Abstract
- Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif: S. K. Saha; EMBO J. 25, 3257 (2006), Abstract
Related Products
| Application | ELISA, IHC, WB |
|---|---|
| Host | Goat |
| Species Reactivity | Rabbit |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?