All members of the inhibitor of apoptosis proteins (IAP) family contain at least one, but usually three copies of baculovirus IAP repeat (BIR), a ~70-aa zinc binding domain, and upon overexpression suppress apoptosis. The BIR motif is capable of interacting with caspases and occluding their catalytic pockets. Certain IAPs also possess C-terminal RING domains. RING-containing proteins often act as E3 ubiquitin ligases and can catalyse the degradation of both, themselves and selected target proteins through ubiquitylation. So far, eight human IAPs have been identified: Apollon (Bruce), c-IAP1 (HIAP2; MIHB), c-IAP2 (HIAP1; MIHC), ILP-2 (Ts-IAP), Livin (ML-IAP; KIAP), NAIP, Survivin (TIAP) and XIAP (ILP-1; MIHA). c-IAP1 has been shown to inhibit specific caspases.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
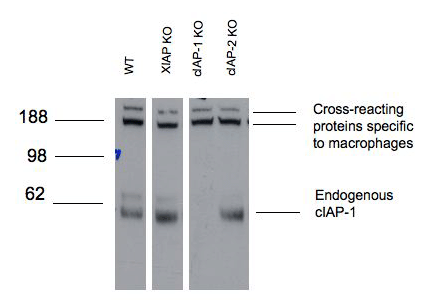
Figure 1: Western blot analysis of c-IAP1 in mouse bone marrow-derived macrophages of the indicated strains lysed in DISC buffer (150mM sodium chloride, 2mM EDTA, 1% Triton X-100, 10% glycerol, 20mM Tris pH 7.5) using MAb to c-IAP1 (1E1-1-10) (Prod. No. ALX-803-335) followed by HRP-anti rat antibody and developed by ECL.Molecular weight marker (kD) is shown at the left. Lanes have been digitally excised from a single blot to aid comparison.
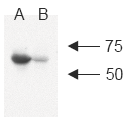
Figure 2: Western blot analysis of human c-IAP1 using MAb to c-IAP1 (1E1-1-10)A: MDA-MB-231 cell lysateB: MDA-MB-231 +LBW 1μM/1h cell lysate (LBW causes degradation of c-IAP1; A. Gaither, et al., 2007)(Image courtesy of Dr. Pascal Meier, The Institute of Cancer Research, London.)
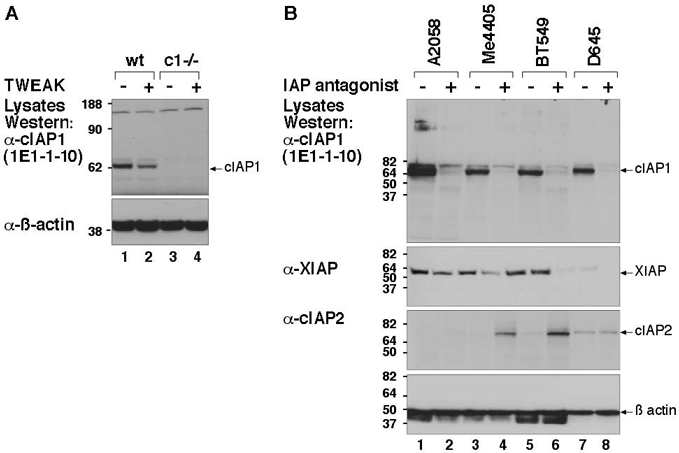
Figure 3: MAb to c-IAP1 (1E1-1-10) is specific for mouse and human c-IAP1. A) Wild type and c-IAP1-/- MEFs were treated +/- TWEAK for 6 hours and whole cell lysates prepared and separated on an SDS-PAGE gel, transferred to a PVDF membrane and probed with MAb to c-IAP1 (1E1-1-10). TWEAK causes a partial loss of c-IAP1. B) 4 human cell lines were treated overnight with an IAP antagonist that induces rapid degradation of c-IAP1. Cells were lysed with DISC lysis buffer and separated on an SDS-PAGE gel, transferred and probed with the MAb to c-IAP1 (1E1-1-10) as in A. XIAP and c-IAP2 blots demonstrate the specificity of the c-IAP1 antibody.
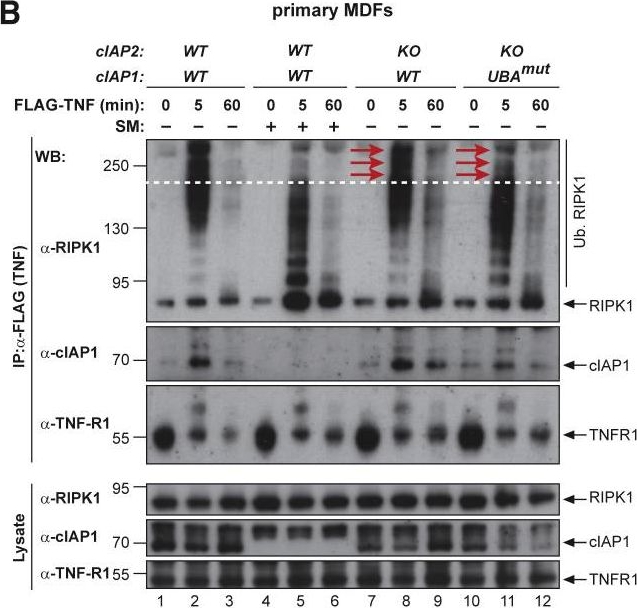
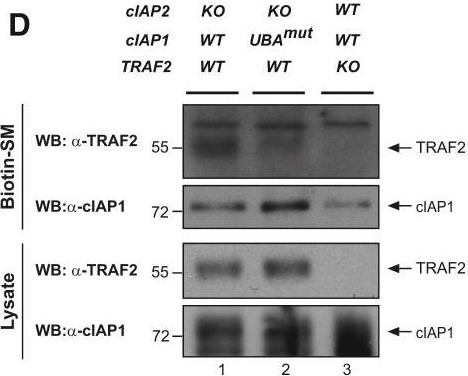
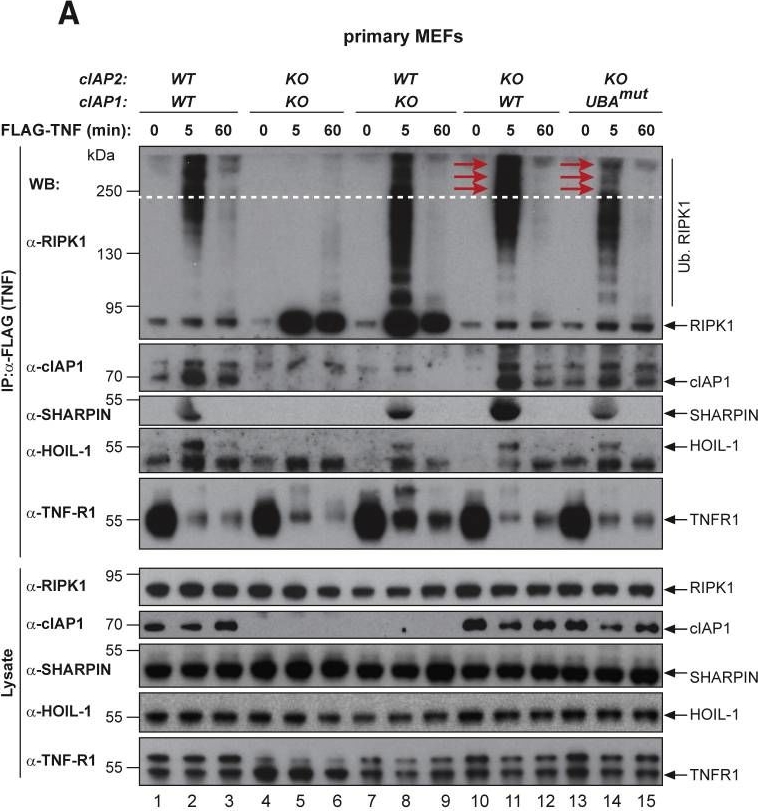
The UBA Directly Regulates RIPK1 Ubiquitylation(A and B) Purification of the TNF-R1 signaling complex (complex-I) from primary MEFs (A) and MDFs (B). Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of three independent experiments are shown.(C) Purification of the TNF-R1 signaling complex (complex-I) from immortalized cIAP2−/− and cIAP1UBAmut MEFs reconstituted either with empty vector (control) or a doxycycline-inducible construct encoding cIAP1UBAmut.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
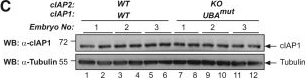
Mice with a Knockin Mutation in the UBA Domain Develop Normally and Do Not Exhibit Defects in the Canonical and Non-canonical NF-κB Activation(A) Gene targeting strategy for the generation of mice with conditional deletion of cIAP2 and conditional mutation of the UBA domain of cIAP1. Exon 2 and 3 of cIAP2 were flanked by FRT sites. To generate the UBA mutation, M396 and F398 were mutated to A396 and A398, respectively. A targeting vector containing a lox-P flanked-minigene spanning exon 4 to 7 of cIAP1 followed by a stop sequence and a hygromycin resistance sequence was used to ensure the WT expression of cIAP1 and therefore the conditional expression of the UBA mutation.(B) Expected and observed numbers of mice from crosses with the respective genotypes.(C) Western blot analysis of cIAP1 protein levels of WT and cIAP1UBAmut MEFs obtained from three different embryos.(D) Biotinylated SM was used to purify IAPs from lysates of cIAP2−/− and cIAP1UBAmut MEFs and TRAF2 binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the indicated antibodies.(E) Purification of the TNF-receptor signaling complex (complex-I) from immortalized MEFs. Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of at least three independent experiments are shown.(F) Western blot analysis of cIAP2−/− and cIAP1UBAmut MEFs treated with SM (100 nM) or TWEAK for 6 hr, followed by western blot analysis using the indicated antibodies.(G) Western blot analysis of MEFs with the indicated genotypes treated with TNF and harvested at the indicated times points.(H and I) The presence of relative mRNA levels (H) and cytokines in the culture media (I) of MEFs treated with TNF (10 ng/mL) for the indicated time points were analyzed by RT-PCR and ELISA, respectively. Graphs show mean ± SD; n = 3 independent biological repeats.(J and K) Primary WT and cIAP1UBAmut BMDMs (J) and keratinocytes (K) were treated with TNF (10 ng/mL) for 2 and 6 hr, and mRNA levels of the indicated cytokines were measured by RT-PCR. Graphs show mean ± SD, n = 3 independent biological repeats.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
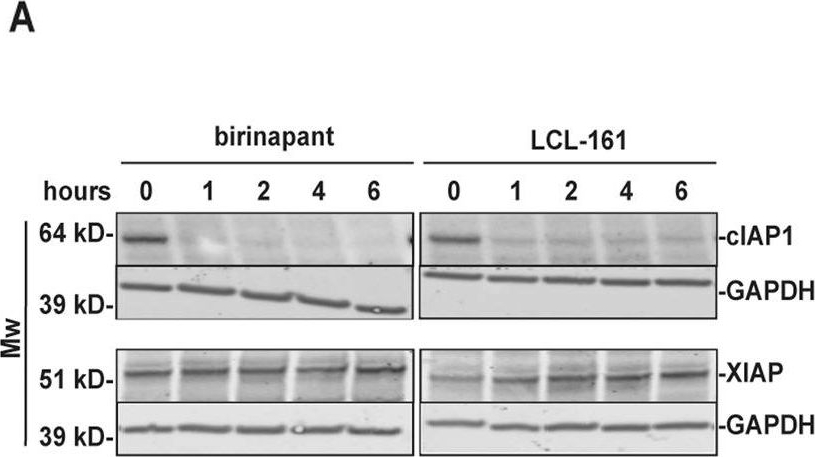
Birinapant and LCL-161 trigger TNF-dependent cell death in pre-OC.Pre-OC were treated with 1 μM birinapant or LCL-161 at the indicated time points, and cell lysates were analyzed for cIAP1 and XIAP protein levels by immunoblotting. GAPDH is loading control, shown for corresponding membranes. One representative of three donors is shown (A). Pre-OC were treated with birinapant (B) or LCL-161 (C) alone or in combination with 25 ng/ml TNF for 18 h and analyzed for cell death. 5 donors, mean, and SD are shown. Pre-OCs were treated with 1 μM of birinapant (D) or LCL-161 (E) in combination with the TNF-blocking antibody infliximab (0.1 μg/ml) and analyzed for cell death. Cell death was measured by LDH-release (B–E), 6 donors, mean and are shown (D–E). Viability of hOCP after treatment with indicated concentrations of LCL-161 (F) and birinapant (G) alone or in combination with 25 ng/ml TNF for 18 h. Results are technical triplicates from one experiment. Cell viability was measured with the Cell Titer Glo assay. Asterisks indicate groups significantly different from control (B, C, F, G) or between indicated groups (D, E, F, G) (p < 0.05, One-way ANOVA).
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Smac-mimetics reduce numbers and viability of human osteoclasts. Cell Death Discov (2021)
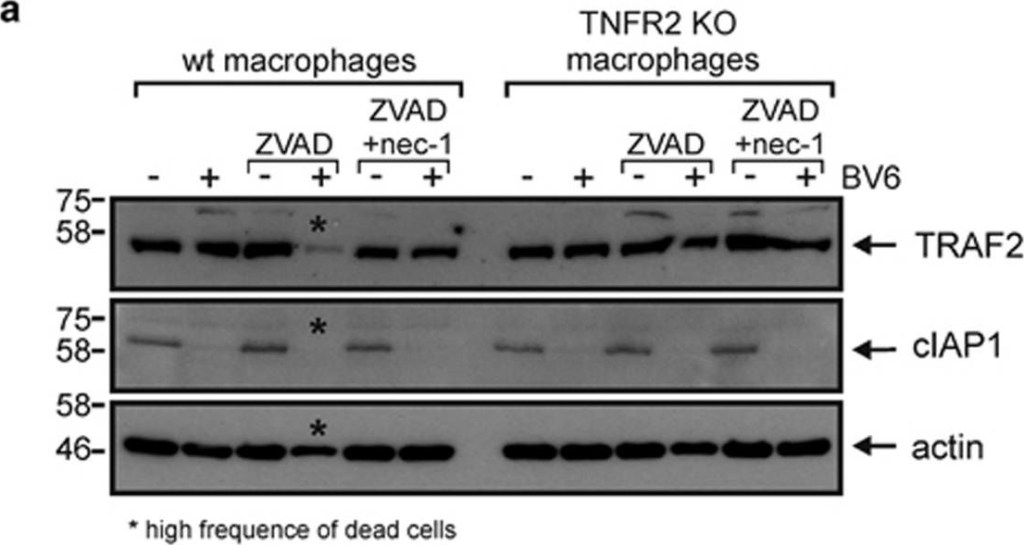
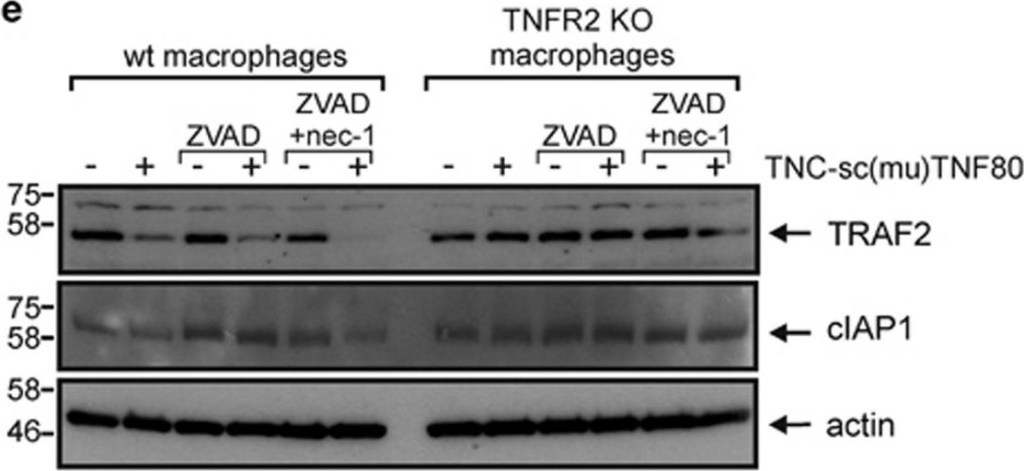
BV6 induces necroptosis in murine macrophages. (a) Wild type and TNFR2-deficient macrophages derived from HoxB8-immortalized MPCs were challenged for 7 h with the indicated combinations of BV6 (10 μM), zVAD-fmk (20 μM) and necrostatin-1 (45 μM). Cells were finally analyzed by western blotting for the presence of TRAF2 and cIAP1. Please note, wild-type cells treated with BV6 and zVAD-fmk were already largely dead when cells were harvested for Western blot analysis. (b) Macrophages derived from HoxB8-immortalized MPCs were stimulated in triplicates (technical replicates) with the indicated concentrations of BV6 in the presence and absence of 20 μM zVAD-fmk and analyzed for viability after 36 h. One of four representative experiments is shown. (c) HoxB8-immortalized MPC-derived macrophages were challenged with the indicated mixtures of 10 μM BV6, 20 μM ZVAD-fmk and 45 μM necrostatin-1 and analyzed for viability after 36 h. Shown are data points with S.E.M. of five independent experiments. (d–f) Macrophages derived from Hoxb8 immortalized MPCs of wild type, TNF- (d), TNFR1- (e) and TNFR2-deficient mice (f) were stimulated with the indicated combinations of BV6 (10 μM) and zVAD-fmk (20 μM). After 36 hours cell viability was quantified using MTT assay or crystal violet staining. Data points derived from six (d and e) or four (f) independent experiments together with mean±S.E.M. are depicted. ***p<0.001
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Activation of TNFR2 sensitizes macrophages for TNFR1-mediated necroptosis. Cell Death Dis (2016)
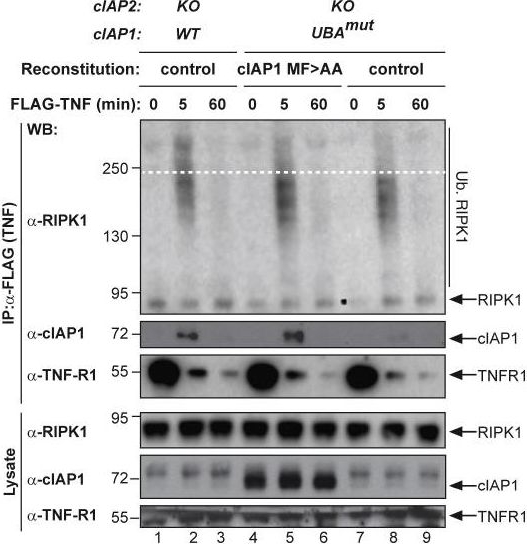
The UBA Directly Regulates RIPK1 Ubiquitylation(A and B) Purification of the TNF-R1 signaling complex (complex-I) from primary MEFs (A) and MDFs (B). Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of three independent experiments are shown.(C) Purification of the TNF-R1 signaling complex (complex-I) from immortalized cIAP2−/− and cIAP1UBAmut MEFs reconstituted either with empty vector (control) or a doxycycline-inducible construct encoding cIAP1UBAmut.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
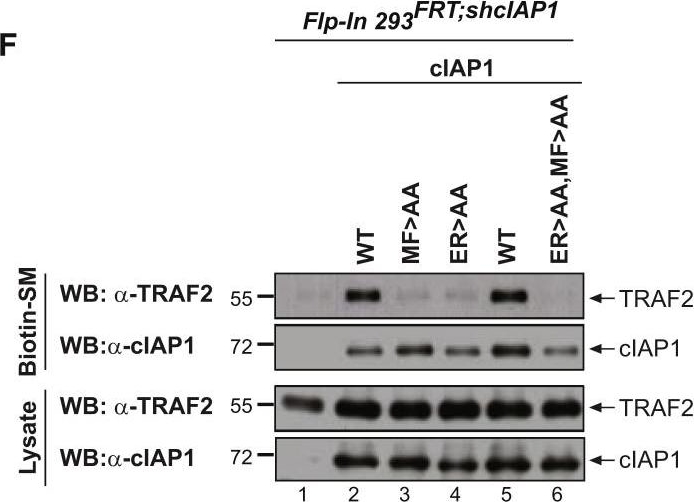
cIAP2 Requires a Functional UBA Domain to Efficiently Interact with TRAF2(A–C) Binding of the indicated cIAP2 fragments to TRAF2 was measured by isothermal titration calorimetry. KD, binding constant. Note the data shown in (A) are from Zheng et al. (2010) and are shown for the purposes of comparison only.(D) Schematic diagram of the Flp-InTMT-RExTM-HEK293shcIAP1 cell system in which endogenous cIAP1 was knocked down via inducible expression of mir30-based shRNA targeting cIAP1’s 3′ UTR. These cells also carry a single FRT site that allows Flp-mediated integration of transgenes into the same transcriptionally regulatable genomic locus. Expression of the transgene and the mir30-based shcIAP1 are induced following treatment with doxycycline (Dox). TRE, tetracycline response element; UBC, ubiquitin promoter; FRT, flippase recognition target; Tet Op, tetracycline operator; Tet-R, tet repressor protein; rtTA3, reverse Tet transactivator (rtTA3).(E) Western blot analysis of Flp-In cells treated for 72 hr with Dox (100 ng/mL), to allow expression of the indicated transgenes, followed by treatment with the SMAC mimetic (SM) compound A (100 nM) for 6 hr.(F and G) Biotinylated SM was used to purify IAPs from lysates of Flp-In cells that were treated with Dox for 72 hr. TRAF2-binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the respective antibodies. Representative immunoblots are shown of three independent experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
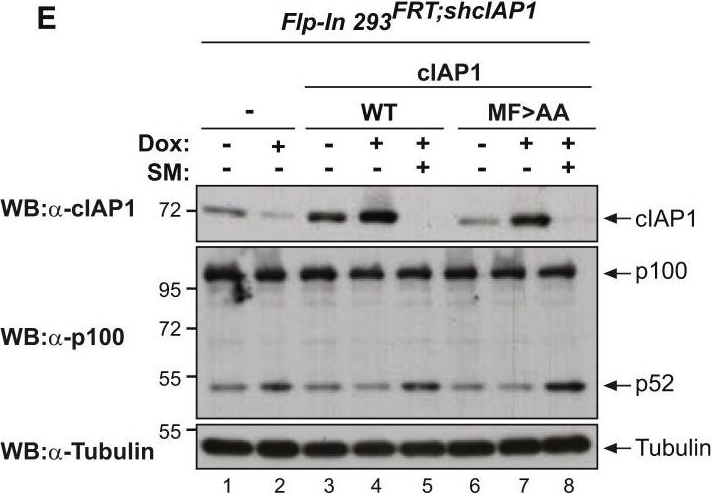
Mice with a Knockin Mutation in the UBA Domain Develop Normally and Do Not Exhibit Defects in the Canonical and Non-canonical NF-κB Activation(A) Gene targeting strategy for the generation of mice with conditional deletion of cIAP2 and conditional mutation of the UBA domain of cIAP1. Exon 2 and 3 of cIAP2 were flanked by FRT sites. To generate the UBA mutation, M396 and F398 were mutated to A396 and A398, respectively. A targeting vector containing a lox-P flanked-minigene spanning exon 4 to 7 of cIAP1 followed by a stop sequence and a hygromycin resistance sequence was used to ensure the WT expression of cIAP1 and therefore the conditional expression of the UBA mutation.(B) Expected and observed numbers of mice from crosses with the respective genotypes.(C) Western blot analysis of cIAP1 protein levels of WT and cIAP1UBAmut MEFs obtained from three different embryos.(D) Biotinylated SM was used to purify IAPs from lysates of cIAP2−/− and cIAP1UBAmut MEFs and TRAF2 binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the indicated antibodies.(E) Purification of the TNF-receptor signaling complex (complex-I) from immortalized MEFs. Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of at least three independent experiments are shown.(F) Western blot analysis of cIAP2−/− and cIAP1UBAmut MEFs treated with SM (100 nM) or TWEAK for 6 hr, followed by western blot analysis using the indicated antibodies.(G) Western blot analysis of MEFs with the indicated genotypes treated with TNF and harvested at the indicated times points.(H and I) The presence of relative mRNA levels (H) and cytokines in the culture media (I) of MEFs treated with TNF (10 ng/mL) for the indicated time points were analyzed by RT-PCR and ELISA, respectively. Graphs show mean ± SD; n = 3 independent biological repeats.(J and K) Primary WT and cIAP1UBAmut BMDMs (J) and keratinocytes (K) were treated with TNF (10 ng/mL) for 2 and 6 hr, and mRNA levels of the indicated cytokines were measured by RT-PCR. Graphs show mean ± SD, n = 3 independent biological repeats.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
cIAP2 Requires a Functional UBA Domain to Efficiently Interact with TRAF2(A–C) Binding of the indicated cIAP2 fragments to TRAF2 was measured by isothermal titration calorimetry. KD, binding constant. Note the data shown in (A) are from Zheng et al. (2010) and are shown for the purposes of comparison only.(D) Schematic diagram of the Flp-InTMT-RExTM-HEK293shcIAP1 cell system in which endogenous cIAP1 was knocked down via inducible expression of mir30-based shRNA targeting cIAP1’s 3′ UTR. These cells also carry a single FRT site that allows Flp-mediated integration of transgenes into the same transcriptionally regulatable genomic locus. Expression of the transgene and the mir30-based shcIAP1 are induced following treatment with doxycycline (Dox). TRE, tetracycline response element; UBC, ubiquitin promoter; FRT, flippase recognition target; Tet Op, tetracycline operator; Tet-R, tet repressor protein; rtTA3, reverse Tet transactivator (rtTA3).(E) Western blot analysis of Flp-In cells treated for 72 hr with Dox (100 ng/mL), to allow expression of the indicated transgenes, followed by treatment with the SMAC mimetic (SM) compound A (100 nM) for 6 hr.(F and G) Biotinylated SM was used to purify IAPs from lysates of Flp-In cells that were treated with Dox for 72 hr. TRAF2-binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the respective antibodies. Representative immunoblots are shown of three independent experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
The UBA Directly Regulates RIPK1 Ubiquitylation(A and B) Purification of the TNF-R1 signaling complex (complex-I) from primary MEFs (A) and MDFs (B). Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of three independent experiments are shown.(C) Purification of the TNF-R1 signaling complex (complex-I) from immortalized cIAP2−/− and cIAP1UBAmut MEFs reconstituted either with empty vector (control) or a doxycycline-inducible construct encoding cIAP1UBAmut.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
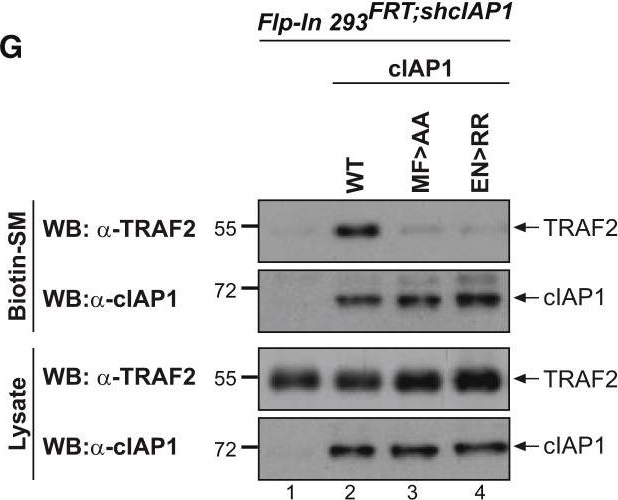
cIAP2 Requires a Functional UBA Domain to Efficiently Interact with TRAF2(A–C) Binding of the indicated cIAP2 fragments to TRAF2 was measured by isothermal titration calorimetry. KD, binding constant. Note the data shown in (A) are from Zheng et al. (2010) and are shown for the purposes of comparison only.(D) Schematic diagram of the Flp-InTMT-RExTM-HEK293shcIAP1 cell system in which endogenous cIAP1 was knocked down via inducible expression of mir30-based shRNA targeting cIAP1’s 3′ UTR. These cells also carry a single FRT site that allows Flp-mediated integration of transgenes into the same transcriptionally regulatable genomic locus. Expression of the transgene and the mir30-based shcIAP1 are induced following treatment with doxycycline (Dox). TRE, tetracycline response element; UBC, ubiquitin promoter; FRT, flippase recognition target; Tet Op, tetracycline operator; Tet-R, tet repressor protein; rtTA3, reverse Tet transactivator (rtTA3).(E) Western blot analysis of Flp-In cells treated for 72 hr with Dox (100 ng/mL), to allow expression of the indicated transgenes, followed by treatment with the SMAC mimetic (SM) compound A (100 nM) for 6 hr.(F and G) Biotinylated SM was used to purify IAPs from lysates of Flp-In cells that were treated with Dox for 72 hr. TRAF2-binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the respective antibodies. Representative immunoblots are shown of three independent experiments.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)
TNF and TNFR1 are required for zVAD-fmk/TNC-sc(mu)TNF80 induced cell death. (a) MPCs and macrophages derived thereof were analyzed by flow cytometry with for cell surface expression of the indicated proteins. (b,c) MPCs and macrophages derived from wild type, TNF- (b) and TNFR1-deficient mice (c) were stimulated with the indicated combinations of human TNF (50 ng/ml), TNC-sc(mu)TNF80 (200 ng/ml) and zVAD-fmk (20 μM). After 36 hours cell viability was quantified using the MTT assay or crystal violet staining. Data points derived from 9 (b) or 7 (c) independent experiments together with with mean±S.E.M. are depicted. (d) Macrophages derived from HoxB8-immortalized MPCs of wild type and TNFR2-deficient mice were stimulated overnight with the indicated combinations of TNC-sc(mu)TNF80 (200 ng/ml) and zVAD-fmk (20 μM). Tnf mRNA induction was analyzed by qPCR. Data points of four independent experiments together with mean±S.E.M. are shown. (e) Wild type and TNFR2-deficient macrophages were treated as indicated for 7 h with TNC-sc(mu)TNF80 (200 ng/ml), zVAD-fmk (20 μM) and necrostatin-1 (45 μM). Cells were analyzed by western blotting for the presence of TRAF2 and cIAP1. ***p<0.001
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Activation of TNFR2 sensitizes macrophages for TNFR1-mediated necroptosis. Cell Death Dis (2016)

Mice with a Knockin Mutation in the UBA Domain Develop Normally and Do Not Exhibit Defects in the Canonical and Non-canonical NF-κB Activation(A) Gene targeting strategy for the generation of mice with conditional deletion of cIAP2 and conditional mutation of the UBA domain of cIAP1. Exon 2 and 3 of cIAP2 were flanked by FRT sites. To generate the UBA mutation, M396 and F398 were mutated to A396 and A398, respectively. A targeting vector containing a lox-P flanked-minigene spanning exon 4 to 7 of cIAP1 followed by a stop sequence and a hygromycin resistance sequence was used to ensure the WT expression of cIAP1 and therefore the conditional expression of the UBA mutation.(B) Expected and observed numbers of mice from crosses with the respective genotypes.(C) Western blot analysis of cIAP1 protein levels of WT and cIAP1UBAmut MEFs obtained from three different embryos.(D) Biotinylated SM was used to purify IAPs from lysates of cIAP2−/− and cIAP1UBAmut MEFs and TRAF2 binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the indicated antibodies.(E) Purification of the TNF-receptor signaling complex (complex-I) from immortalized MEFs. Cells of the indicated genotypes were treated with FLAG-TNF for 0, 5, and 60 min. Cell lysates were then subjected to FLAG IP followed by western blot analysis with the indicated antibodies. Representative images of at least three independent experiments are shown.(F) Western blot analysis of cIAP2−/− and cIAP1UBAmut MEFs treated with SM (100 nM) or TWEAK for 6 hr, followed by western blot analysis using the indicated antibodies.(G) Western blot analysis of MEFs with the indicated genotypes treated with TNF and harvested at the indicated times points.(H and I) The presence of relative mRNA levels (H) and cytokines in the culture media (I) of MEFs treated with TNF (10 ng/mL) for the indicated time points were analyzed by RT-PCR and ELISA, respectively. Graphs show mean ± SD; n = 3 independent biological repeats.(J and K) Primary WT and cIAP1UBAmut BMDMs (J) and keratinocytes (K) were treated with TNF (10 ng/mL) for 2 and 6 hr, and mRNA levels of the indicated cytokines were measured by RT-PCR. Graphs show mean ± SD, n = 3 independent biological repeats.
Image collected and cropped by CiteAb under a CC-BY license from the following publication: Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol Cell (2018)















Product Details
| Alternative Name |
HIAP2, BIRC-2, Baculoviral IAP repeat-containing protein-2, Cellular inhibitor of apoptosis-1, Human inhibitor of apoptosis protein-2, Inhibitor of apoptosis protein-2 |
|---|---|
| Application |
WB |
| Application Notes |
Detects a band of ~62kDa by Western blot. |
| Clone |
1E1-1-10 |
| Formulation |
Liquid. 0.2μm-filtered solution in PBS containing 0.05% sodium azide. |
| Host |
Rat |
| Immunogen |
Synthetic peptide corresponding to aa 221-232 of mouse c-IAP1. |
| Isotype |
IgG2a |
| Positive Control |
Included. (ALX-803-335-POS. Wild type MEF lysate in SDS loading buffer). Negative control also included (ALX-803-335-NEG. c-Iap1-/- MEF lysate in SDS loading buffer.) |
| Recommendation Dilutions/Conditions |
Western Blot (1:1,000-1:4,000 using ECL; suggested blocking and dilution buffer is PBST containing 0.1% Tween 20 and either 5% skim milk or 5% BSA. Suggested incubation time is 1 hour at room temperature or overnight at 4°C.)Suggested dilutions/conditions may not be available for all applications.Optimal conditions must be determined individually for each application. |
| Species Reactivity |
Human, Mouse |
| UniProt ID |
Q62210 |
| Worry-free Guarantee |
This antibody is covered by our Worry-Free Guarantee. |
Handling & Storage
| Use/Stability |
Stable for at least 12 months after receipt when stored at -80°C. |
|---|---|
| Handling |
Avoid freeze/thaw cycles. After opening, prepare aliquots and store at -80°C. |
| Short Term Storage |
+4°C |
| Long Term Storage |
-80°C |
| Shipping |
Dry Ice |
| Regulatory Status |
RUO – Research Use Only |
|---|
- O-GlcNAcylation of RIPK1 rescues red blood cells from necroptosis.: Seo, J., Kim, Y., et al.; Front. Immunol. 14, 1160490 (2023), Application(s): WB, Abstract
- SMAC Mimetics Synergistically Cooperate with HDAC Inhibitors Enhancing TNF-α Autocrine Signaling.: Shibuya, Y., Kudo, K., et al.; Cancers (Basel) 15, (2023), Application(s): WB, Abstract
- Protease-independent control of parthanatos by HtrA2/Omi.: Weiß, J., Heib, M., et al.; Cell. Mol. Life Sci. 80, 258 (2023), Application(s): WB / Reactant(s): Mouse, Abstract
- A1 is induced by pathogen ligands to limit myeloid cell death and NLRP3 inflammasome activation.: Speir, M., Tye, H., et al.; EMBO Rep. 24, e56865 (2023), Application(s): WB, Abstract
- A RIPK1-specific PROTAC degrader achieves potent antitumor activity by enhancing immunogenic cell death: F. Saleem, et al.; Microorganisms 11, 851 (2023), Abstract
- Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation: Zhang, T., Xu, D., et al.; Nat. Cell Biol. 25, 950 (2023), Abstract
- Human ZBP1 induces cell death-independent inflammatory signaling via RIPK3 and RIPK1.: Peng, R., Wang, C. K., et al.; EMBO Rep. 23, e55839 (2022), Application(s): WB / Reactant(s): SARS-CoV-2 coronavirus, Abstract
- Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway: Simpson, D. S., Pang, J., et al.; Immunity 55, 423 (2022), Abstract
- XIAP promotes melanoma growth by inducing tumour neutrophil infiltration: M. Daoud, et al.; EMBO Rep. 23, e53608 (2022), Application(s): WB, Abstract
- ZBP1 induces inflammatory signaling via RIPK3 and promotes SARS-CoV-2-induced cytokine expression: Peng, R., Wang-Kan, X., et al.; bioRxiv , (2021)
- Clinical stage drugs targeting inhibitor of apoptosis proteins purge episomal Hepatitis B viral genome in preclinical models.: Mahmoudi, T., Pellegrini, M., et al.; Cell Death Dis. 12, 641 (2021), Application(s): IHC, Abstract
- Smac-mimetics reduce numbers and viability of human osteoclasts.: Starheim, K. K., Nonstad, U., et al.; Cell Death Discov. 7, 36 (2021), Application(s): WB / Reactant(s): Mouse, Abstract
- Impaired RIPK1 ubiquitination sensitizes mice to TNF toxicity and inflammatory cell death.: Goncharov, T., Vucic, D., et al.; Cell Death Differ. 28, 985 (2021), Application(s): WB / Reactant(s): Mouse, Abstract
- Clinical Positioning of the IAP Antagonist Tolinapant (ASTX660) in Colorectal Cancer: Crawford, N., Stott, K. J., et al.; Mol. Cancer Ther. 20, 1627 (2021), Abstract
- Diversity of cell death signaling pathways in macrophages upon infection with modified vaccinia virus Ankara (MVA): Klaas, L., Vier, J., et al.; Cell Death Dis. 12, 1011 (2021), Abstract
- Overlap of NatA and IAP substrates implicates N-terminal acetylation in protein stabilization: Müller, F., Friese, A., et al.; Sci. Adv. 7, (2021), Abstract
- Regulation of CYLD activity and specificity by phosphorylation and ubiquitin-binding CAP-Gly domains: P.R. Elliott, et al.; Cell Rep. 37, 109777 (2021), Application(s): WB / Reactant(s) Human, Abstract
- An incoherent feedforward loop interprets NFκB/RelA dynamics to determine TNF-induced necroptosis decisions: Oliver Metzig, M., Tang, Y., et al.; Mol. Syst. Biol. 16, e9677 (2020), Abstract
- Ubiquitin Ligases cIAP1 and cIAP2 Limit Cell Death to Prevent Inflammation: J. Zhang, et al.; Cell Rep. 27, 2679 (2019), Application(s): WB / Reactant(s) Mouse, Abstract
- Targeting RIPK1 in AML cells carrying FLT3-ITD.: Hillert, L. K., Bettermann-Bethge, K., et al.; Int. J. Cancer 145, 1558 (2019), Application(s): WB, Abstract
- RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development.: Anderton, H., Bandala-Sanchez, E., et al.; Cell Death Differ. 26, 877 (2019), Application(s): WB / Reactant(s): Mouse, Abstract
- A20 protects cells from TNF-induced apoptosis through linear ubiquitin-dependent and -independent mechanisms: Priem, D., Devos, M., et al.; Cell Death Dis. 10, 692 (2019), Abstract
- BAX/BAK-Induced Apoptosis Results in Caspase-8-Dependent IL-1β Maturation in Macrophages: D. Chauhan, et al.; Cell Rep. 25, 2354 (2018), Abstract
- Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2: A. Annibaldi, et al.; Mol. Cell 69, 566 (2018), Application(s): WB / Reactant(s) Mouse, Abstract — Full Text
- Heat Shock Protein 70 (Hsp70) Suppresses RIP1-Dependent Apoptotic and Necroptotic Cascades: S.R. Srinivasan, et al.; Mol. Cancer Res. 16, 58 (2018), Application(s): WB / Reactant(s) Human, Abstract — Full Text
- X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition: L.C. Cesa, et al.; J. Biol. Chem. 293, 2370 (2018), Abstract — Full Text
- The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation.: Vince, J. E., De Nardo, D., et al.; Cell Rep. 25, 2339 (2018), Application(s): WB / Reactant(s): Mouse, Abstract
- A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival.: Kattah, M. G., Shao, L., et al.; J. Exp. Med. 215, 1839 (2018), Application(s): WB, Abstract
- The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts.: Armaka, M., Ospelt, C., et al.; Nat. Commun. 9, 618 (2018), Application(s): WB, Abstract
- Polygalacin D induces apoptosis and cell cycle arrest via the PI3K/Akt pathway in non-small cell lung cancer.: Seo, Y. S., Kang, O. H., et al.; Oncol. Rep. 39, 1702 (2018), Reactant(s): Human, Abstract
- Disruption of XIAP-RIP2 Association Blocks NOD2-Mediated Inflammatory Signaling: T. Goncharov, et al.; Mol. Cell 69, 551 (2018), Reactant(s) Human, Abstract
- Exploration of Benzothiazole Rhodacyanines as Allosteric Inhibitors of Protein-Protein Interactions with Heat Shock Protein 70 (Hsp70): Shao, H., Li, X., et al.; J. Med. Chem. 61, 6163 (2018), Abstract
- Necroptotic signaling is primed in Mycobacterium tuberculosis-infected macrophages, but its pathophysiological consequence in disease is restricted: Stutz, M. D., Ojaimi, S., et al.; Cell Death Differ. 25, 951 (2018), Abstract
- SPATA2 regulates the activation of RIPK1 by modulating linear ubiquitination.: Yuan, J., Zhu, H., et al.; Genes Dev. 31, 1162 (2017), Application(s): WB, Abstract
- Mitochondrial permeabilisation engages NF-κB dependent anti-tumour activity under caspase deficiency: E. Giampazolias, et al.; Nat. Cell Biol. 19, 1116 (2017), Application(s): WB / Reactant(s) Mouse, Abstract
- Pentamidine blocks hepatotoxic injury in mice: E. Zhao, et al.; Hepatology 66, 922 (2017), Application(s): WB, Abstract — Full Text
- In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs): Ohoka, N., Okuhira, K., et al.; J. Biol. Chem. 292, 4556 (2017), Abstract
- MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death: I. Jaco, et al.; Mol. Cell 66, 698 (2017), Application(s): WB / Reactant(s) Mouse, Abstract — Full Text
- Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death: M.C. de Almagro, et al.; Cell Death Differ. 24, 26 (2017), Application(s): Western Blot, Abstract — Full Text
- Activation of TNFR2 sensitizes macrophages for TNFR1-mediated necroptosis: D. Siegmund, et al.; Cell Death Dis. 7, e2375 (2016), Application(s): WB / Reactant(s) Mouse, Abstract — Full Text
- A critical role for cellular inhibitor of protein 2 (cIAP2) in colitis-associated colorectal cancer and intestinal homeostasis mediated by the inflammasome and survival pathways: M. Dagenais, et al.; Mucosal Immunol. 9, 146 (2016), Abstract
- Knockdown of RIPK1 Markedly Exacerbates Murine Immune-Mediated Liver Injury through Massive Apoptosis of Hepatocytes, Independent of Necroptosis and Inhibition of NF-κB: Suda, J., Dara, L., et al.; J. Immunol. 197, 3120 (2016), Abstract
- Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination: M.C. de Almagro, et al.; Cell Death Dis. 6, e1800 (2015), Abstract — Full Text
- Effects of physiological and synthetic IAP antagonism on c-IAP-dependent signaling: A.J. Kocab, et al.; Oncogene 34, 5472 (2015), Abstract
- RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL: K.E. Lawlor, et al.; Nat. Commun. 6, 6282 (2015), Application(s): WB, Abstract — Full Text
- Ubiquitin-dependent regulation of MEKK2/3-MEK5-ERK5 signaling module by XIAP and cIAP1.: Takeda, A. N., Oberoi-Khanuja, T. K., et al.; EMBO J. 33, 1784 (2014), Application(s): WB / Reactant(s): Human, Abstract
- RIPK1 promotes death receptor-independent caspase-8-mediated apoptosis under unresolved ER stress conditions.: Estornes, Y., Aguileta, M. A., et al.; Cell Death Dis. 5, e1555 (2014), Abstract
- RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis.: Polykratis, A., Pasparakis, M., et al.; Nature 513, 90 (2014), Application(s): WB / Reactant(s): Mouse, Abstract
- Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells: Antonopoulos, C., El Sanadi, C., et al.; J. Immunol. 191, 4789 (2013), Abstract
- RANKL enhances the effect of an antagonist of inhibitor of apoptosis proteins (cIAPs) in RANK-positive breast cancer cells: Casimiro, S., Alho, I., et al.; J. Bone Oncol. 2, 116 (2013), Abstract
- c-IAP1 binds and processes PCSK9 protein: linking the c-IAP1 in a TNF-α pathway to PCSK9-mediated LDLR degradation pathway: Xu, W., Liu, L., et al.; Molecules 17, 12086 (2012), Abstract
- An inactivating caspase 11 passenger mutation originating from the 129 murine strain in mice targeted for c-IAP1: N.S. Kenneth, et al.; Biochem. J. 443, 355 (2012), Application(s): WB using mouse embryonic fibroblast (MEF) cell lysate, Abstract — Full Text
- Molecular determinants of Smac mimetic induced degradation of cIAP1 and cIAP2: M. Darding, et al.; Cell Death Differ. 18, 1376 (2011), Abstract — Full Text
- TNF signaling, but not TWEAK triggered cellular Inhibitor of Apoptosis protein 1 (cIAP1) degradation, requires cIAP1 RING dimerization and E2 binding: R. Feltham, et al.; J. Biol. Chem. 285, 17525 (2010), Abstract — Full Text
- TAK1 is required for survival of mouse fibroblasts treated with TRAIL, and does so by NF-kappaB dependent induction of cFLIPL: J.M. Lluis, et al.; PLoS One 5, e8620 (2010), Abstract — Full Text
- Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment: P. Geserick, et al.; J. Cell Biol. 187, 1037 (2009), Abstract — Full Text
- TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis: J.E. Vince, et al.; J. Biol. Chem. 284, 35906 (2009), Reactant(s) Mouse, Abstract
- TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha: J.E. Vince, et al.; J. Cell. Biol. 182, 171 (2008), Abstract — Full Text
- IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis: J.E. Vince, et al.; Cell 131, 682 (2007), Abstract
- A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling: A. Gaither, et al.; Cancer Res. 67, 11493 (2007), Abstract — Full Text
Related Products

| Alternative Name | HIAP-1, BIRC3, Baculoviral IAP repeat-containing protein-3, Cellular inhibitor of apoptosis-2, Human inhibitor of apoptosis protein-1, RNF49, API2 |
|---|---|
| Application | WB |
| Host | Rat |
| Isotype | IgG1 |
| Species Reactivity | Human |
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
