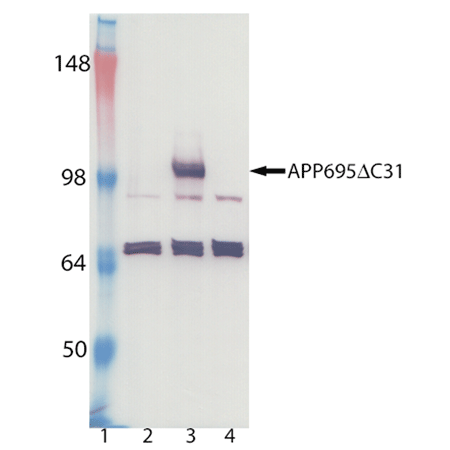
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by the senile plaques, neurofibrillary tangles and loss of synapses and neurons. AD has been largely viewed as a disease of toxicity being mediated by the accumulation of the amyloid beta (Aβ) peptide as plaques within the brain resulting in damage to brain cells from the binding of damaging metals, reactive oxygen species production and direct damage to cellular membranes. Recent research has suggested that the Aβ peptide is a multifunctional peptide with non-pathological effects and that its association with AD is in conjunction with its roles in combination with other proteins such as the amyloid precursor protein (APP) resulting in the imbalance between the processes of memory formation and normal forgetting. It is through the interactions of the Aβ peptide with APP that the Aβ peptide itself can affect normal modulation and signaling of APP resulting in its indicated role in the pathogenesis of AD via signaling effects rather than chemical or physical effects. There are three major APP isoforms (APP695, APP751 and APP770) that are formed through alternative splicing of precursor mRNA. APP770 represents the canonical sequence. The APP695 isoform is preferentially expressed in the central nervous system, while APP770 and APP751 are more highly expressed in peripheral tissues. It has been demonstrated that the full length APP695 can be cleaved via caspase at an intracellular site (Asp664) resulting in the release of a 31 amino acid C-terminal peptide (C31) from the remaining larger neo-APP fragment (APP ΔC31) with both of these entities being pro-apoptotic. Immunohistochemical analysis of human brain tissue demonstrated that this cytoplasmic cleavage occurs 4-fold greater in patients with AD versus normal patients and that these cleavage products are localized to plaques and tangles in key areas of the brain affected by the disease. A single genetic mutation of aspartic acid residue 664 to alanine of APP695 led to the complete blockage of the C-terminal cleavage in vivo reversing many characteristics of the AD phenotype in a transgenic mouse model. Additionally, in cell culture it has been suggested that the neurotoxicity of Aβ is dependent on the cleavage of APP at Asp664 and the resulting Aβ-facilitated APP multimerization.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?