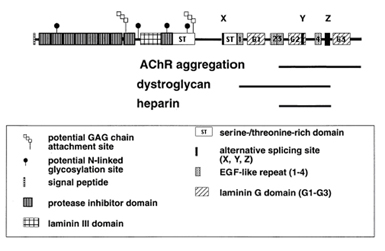
Agrin is an essential extracellular matrix component which promotes clustering of nicotinic acetylcholine receptors (nAChRs) and other proteins during development at the neuromuscular junction. Agrin, MuSK and Rapsyn are all essential components for AChR aggregation through an unknown mechanism. The C-terminal region of agrin is released into the medium, interacts with receptors on the muscle surface, and induces AChR aggregation. The central region contains two O-linked glycosylation sites and a domain homologous to domain III of laminin. The N-terminal region anchors agrin to the extracellular matrix via other basal membrane components. This region also contains a protease inhibitor domain and glycosaminoglycan attachment sites, increasing the predicted MW from 200kDa to ~600kDa. The diagram shown indicates the domain structure and functional regions of agrin, as well as domains required for AChR aggregation and alpha-dystroglycan and heparin binding. Various agrin isoforms are generated by alternative splicing at the X, Y and Z sites, and differ in the presence or absence of small inserts. The isoforms can determine the biological activity of agrin and their expression in specific tissues and stages of development. While no difference in functional activity has been detected between splicing variants at site X, insertion of a 4 aa peptide at site Y modestly increases agrins nAChR clustering activity. Insertion of an 8 aa peptide at splicing site Z increases the clustering activity of soluble rat agrin 10,000 fold.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
This antibody is covered by our Worry-Free Guarantee.
| Regulatory Status |
RUO – Research Use Only |
|---|
Last modified: May 29, 2024
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?