Thymidylate synthetase inhibitor
Potent antitumor agent. Inhibits thymidylate synthetase. Induces p53-dependent apoptosis.
Shipping: Available products typically ship within 24/48h, via priority shipping.
Do you need support? Contact Customer Service or Technical Support.
Online Account
Access or Create Your Account
Product Details
| Alternative Name |
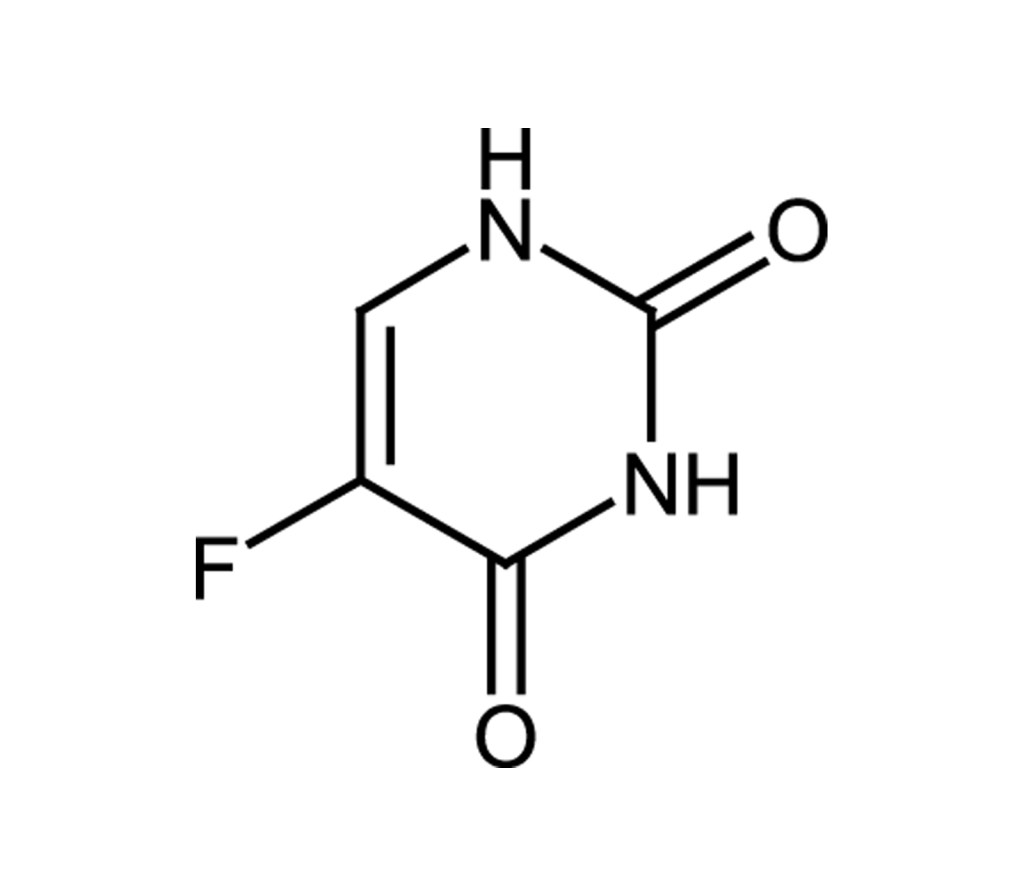
5-FU, 2,4-Dioxo-5-fluoropyrimidine, 5-Fluoro-2,4(1H,3H)-pyrimidinedione |
|---|---|
| Appearance |
White to off-white crystalline powder. |
| CAS |
51-21-8 |
| Couple Target |
Thymidylate synthetase |
| Couple Type |
Inhibitor |
| Formula |
C4H3FN2O2 |
| Identity |
Determined by IR. |
| MI |
14: 4181 |
| MW |
130.1 |
| Purity |
≥98% (HPLC) |
| RTECS |
YR0350000 |
| Solubility |
Soluble in DMSO (5mg/ml), dimethyl formamide (5mg/ml), methanol (1mg/ml) or hot water (1mg/ml). |
Handling & Storage
| Use/Stability |
As indicated on product label or CoA when stored as recommended. |
|---|---|
| Long Term Storage |
-20°C |
| Shipping |
Ambient Temperature |
| Regulatory Status |
RUO – Research Use Only |
|---|
- CRISPR/Cas9-Mediated Induction of Relapse-Specific NT5C2 and PRPS1 Mutations Confers Thiopurine Resistance as a Relapsed Lymphoid Leukemia Model: Nguyen, T. T. T., Tanaka, Y., et al.; Mol. Pharmacol. 103, 199 (2023), Abstract
- Germline thymidylate synthase deficiency impacts nucleotide metabolism and causes dyskeratosis congenita: H. Tummala, et al.; Am. J. Hum. Genet. 109, 1472 (2022), Abstract
- A selective p53 activator and anticancer agent to improve colorectal cancer therapy: H. Ramos, et al.; Cell Rep. 35, 108982 (2021), Abstract
- PRX1 knockdown potentiates vitamin K3 toxicity in cancer cells: a potential new therapeutic perspective for an old drug: T. He, et al.; J. Exp. Clin. Cancer Res. 34, 152 (2015), Application(s): Cell culture, Abstract — Full Text
- Gonadotropin-mediated chemoresistance: Delineation of molecular pathways and targets: Sahoo, S., Singh, P., et al.; BMC Cancer 15, 931 (2015), Abstract
- Omega-3 eicosapentaenoic acid decreases CD133 colon cancer stem-like cell marker expression while increasing sensitivity to chemotherapy: De Carlo, F., Witte, T. R., et al.; PLoS One 8, e69760 (2013), Abstract
- Tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor growth of human colorectal cancer: He, B. C., Gao, J. L., et al.; Mol. Pharmacol. 79, 211 (2011), Abstract
- 5-fluorouracil sensitivity and dihydropyrimidine dehydrogenase activity in advanced gastric cancer: T. Inada, et al.; Anticancer Res. 20, 2457 (2000), Abstract
- Effect of 5-fluorouracil on cell cycle regulatory proteins in human colon cancer cell line: H. Takeda, et al.; Jpn. J. Cancer Res. 90, 677 (1999), Abstract
- Effects of perturbations of pools of deoxyribonucleoside triphosphates on expression of ribonucleotide reductase, a G1/S transition state enzyme, in p53-mutated cells: S. Wadler, et al.; Biochem. Pharmacol. 55, 1353 (1998), Abstract
- Chemically-induced apoptosis: p21 and p53 as determinants of enterotoxin activity: D.M. Pritchard, et al.; Toxicol. Lett. 102, 19 (1998), Abstract
- Nucleoside-mediated mitigation of 5-fluorouracil-induced toxicity in synchronized murine erythroleukemic cells: K.H. Elstein, et al.; Toxicol. Appl. Pharmacol. 146, 29 (1997), Abstract
- Modulation of the equilibrative nucleoside transporter by inhibitors of DNA synthesis: J. Pressacco, et al.; Br. J. Cancer 72, 939 (1995), Abstract
- Apoptosis of human primary and metastatic colon adenocarcinoma cell lines in vitro induced by 5-fluorouracil, verapamil, and hyperthermia: I.B. Shchepotin, et al.; Anticancer Res. 14, 1027 (1994), Abstract
Last modified: May 29, 2024
Datasheet, Manuals, SDS & CofA
Manuals And Inserts
Certificate of Analysis
Please enter the lot number as featured on the product label
SDS
Enzo Life Science provides GHS Compliant SDS
If your language is not available please fill out the SDS request form
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?