Basics of Immunity
Even though the most sophisticated immunity mechanisms are those of vertebrates, some sort of innate defense is present in all living organisms, including protozoans, fungi, plants, and invertebrate animals.
The immune system of vertebrates is composed of three layers of defense (Figure 1). The first one consists of anatomical barriers, such as the skin and the mucous membranes (e.g., mouth, nose, lung), physically preventing most potential pathogens from reaching the infection site.
If the germ makes it through this first obstacle, it will confront the innate and the adaptive immune system.
Innate immunity represents the first response of the body when an intruder is detected. It is often referred to as “non-specific immunity” because it does not discriminate different pathogens, but rather recognizes specific patterns of the foreign organisms (e.g., CpG motifs, dsRNA, lipopolysaccharide, peptidoglycan, etc.). These cells do not have a memory of the previous battles, so they do not recognize an enemy already defeated and cannot adapt their response. Cells involved in innate immunity act rapidly (within hours), producing cytokines responsible for the inflammatory process and the recruitment of other immune cells into the infection site. They also have a crucial role in eliciting the adaptive response.
Adaptive immunity intervenes when the innate system is not sufficient against an established infection. Opposite to the previous ones, the cells of the adaptive system generate a pathogen-specific immunologic response to eliminate the target micro-organism and/or the infected cells. The cells involved in the adaptive immunity can also develop an immunologic memory, thus rendering the reaction to a second infection faster and more efficient. Vaccine development exploits this principle.

Main actors of the innate immune response
The cells composing the immune system are collectively called leukocytes (or white blood cells). The leukocytes of the innate immunity are classified as granulocytes (neutrophils, basophils, and eosinophils), macrophages, mast cells, dendritic cells (DCs), and Natural Killer Cells (NK).
Neutrophils are phagocytic cells, therefore capable of engulfing and “eating” the target (phagocytosis). Neutrophils are the most abundant leukocytes and are classified as “granulocytes” for the granules visible in their cytoplasm, containing substances toxic to bacteria and fungi.
Basophils and eosinophils protect against multicellular parasites (e.g., worms) and are also mediators of the allergic response, together with mast cells.
Macrophages are the most efficient phagocytes. Capable of leaving the circulatory system squeezing through the walls of capillary vessels, they patrol for pathogens or dead cells. Therefore, macrophages are the first line of defense of the innate immune system eliminating a substantial number of targets. They also have a role in the activation of T cells via the mechanism of antigen presentation (see below).
Mast cells reside in the connective tissue around the blood vessels and mucous membranes. They are typically involved in wound healing and the inflammatory response by releasing chemical mediators, such as histamine and cytokines.
Dendritic Cells are located in tissues in direct contact with the external environment, more susceptible to infections, such as skin, mucosa lining of nose, lungs, intestine, stomach, etc. DCs can easily identify threats, acting as messengers for the rest of the immune cells. They are indeed considered a bridge between innate and adaptive immunity, as they act as antigen presenting cells once the pathogen has been “eaten” by the phagocytic process (similarly to macrophages; see below).
Natural Killer cells do not attack the pathogen, rather induce apoptosis in the infected cell through the Fas signaling pathway or the release of cytotoxic proteins (perforins and granzymes). Even though they are considered innate immunity cells, they share some features with the cells of the adaptive immune system, as the development of memory-like NK cells and the capacity to multiply in response to some viral infections or cancers.
As previously mentioned, cells of innate immunity recognize conserved features in invading pathogens, identified as “non-self” via a family of pattern recognition receptors (PRRs), called Toll-Like Receptors (TLRs). 10 TLR subtypes have been found in humans, binding to different external pathogen-associated molecular patterns (PAMPs).
Each TLR subtype recognizes a class of ligands characterized by a type of feature. For example, TLR3 binds to dsRNA (virus signature), TLR4 binds to LPS (typical of Gram-negative bacteria), TLR8 binds to ssRNA (virus), TLR9 to methylated CpG (indicating the presence of bacteria and virus), etc.
The binding of these receptors to their targets initiates the signaling cascade leading to the inflammatory reaction. Signaling molecules such as cytokines (small secreted cell-impermeable proteins), or eicosanoids (biologically active unsaturated fatty acids lipid compounds) mediate inflammation. These factors will attract immune cells to the infection site causing other inflammatory manifestations (e.g., fever, vasodilation).
Main actors of the adaptive immune response
The cell types of adaptive immune system are Lymphocytes B and Lymphocytes T. These specialized cells express somatically generated, highly diverse, antigen-specific T or B cell Receptors (TCRs or BCRs), produced by random rearrangement of the T cell receptor and Immunoglobulin (Ig) gene segments, respectively. This process allows the generation of a diverse repertoire and a flexible range of responses to pathogens. The adaptive immunity includes the development of memory B cells and memory T cells that trigger a more rapid and extensive response following subsequent antigen exposure.
Activation of Lymphocytes T (or T cells) occurs only upon interaction with Antigen Presenting Cells (APCs). APCs express cell-surface proteins known as the major histocompatibility complex (MHC), classified as class I (found in all nucleated cells) or class II (found for example on macrophages, dendritic cells, and B cells). After engulfing and digesting an intruder, APCs display the antigen fragments on the surface bound to its MHC. The MHC-antigen complex binds to the TCR and activates the T cell.
Unsurprisingly, the TCRs are not the only receptors expressed on T cell membranes. The two major lymphocyte T subsets are distinguished based on the presence of CD4 or CD8 receptors: T-helper cells (Th) express the first (CD4+/CD8- cells), whereas cytotoxic T cells express the second receptor (CD4-/CD8+ cells).
Upon activation via interaction of their TCR with the MHC class II on the APC, Th cells release cytokines influencing the immune response, for example modulating B cell activity.
As suggested by their name, the CD8+ cytotoxic T cells directly destroy the infected cells. Upon their interaction with the MHC class I, clonal cytotoxic T cells differentiate into effector cells, which in turn secrete perforin, granzyme (proteins that cause lysis of target cells), and granulysin (a substance that induces apoptosis of target cells). Some of the CD8+ T cells will persist as memory cells after the resolution of the infection.
The activation process of B cells is quite different as it does not require the APCs mediation. The B Cell Receptor (BCR) complex contains a transmembrane immunoglobulin, acting as a specific antigen-binding receptor. The full activation of the B cell happens through the interaction with a T helper cell activated by the same antigen. This cell-to-cell cross-talk occurs between a protein called the CD40 ligand (CD40L), which appears on the surface of the activated helper T cells, and the CD40 protein on the B-cell surface. Active B cells multiply and mature into plasma cells (or effector B cells), secreting antibodies with the same antigen specificity as the BCR.
The antibody-antigen interaction flags the pathogen for elimination through phagocytosis (scavenger cells recognize better germs loaded with antibodies) or renders it harmless (e.g., neutralizing toxins, immobilization of microorganisms, or preventing cell penetration).
Also, in this case, some of the activated B cells differentiate into long-lived memory cells expressing the same membrane-bound antibody as the original parent B cell.
Tools to study immune response
The paragraphs above describe a simplified vision of the main features of the different immune cells. In reality, each of them express specific combinations of markers, responsible for their interaction and the fine-tuning of their activity. This combination of markers is often exploited by researchers to identify a specific immune population, for example by IHC of Flow Cytometry.
To this aim, Enzo offers a broad range of antibodies for use in all areas of immunology. Find your inspiration in the table below. Remember that every antibody is backed by our Worry-Free Guarantee, allowing you to evaluate any of our antibodies of interest for your specific application or species without risk.
The same molecules that elicit the immune response (toxins, cytokines, recombinant ligands, etc.) are often used in experiments to activate specific cell populations, not only to characterize the mechanisms underlying immunity but also to develop therapies, drug treatments and vaccines.
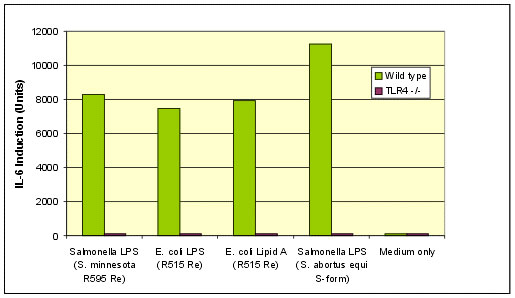
Among the products for Immunology Research, Enzo offers a selection of high purity TLR-specific ligands for the co-stimulation of the immune cells and the study of the immune response mechanism. As an example, the graph in figure 2 compares the activation of macrophages obtained from TLR4 deficient versus wild-type mice, following treatment with Enzo’s LPS or Protein A, from different organisms.
Our bacterial toxins and viral antigens are produced in mammalian expression systems to ensure that they are in their native folded state and possess all relevant post-translational modifications, to deliver optimal antigenicity for use in vaccine therapeutics, serology-based diagnostic assays, cytotoxicity assays, and more. Check out our complete product range here: Native Toxins and Antigens for Diagnostics Development.
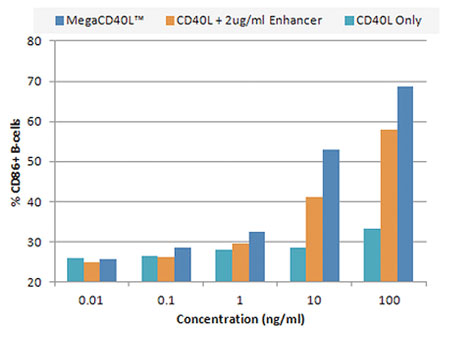
Looking for a high purity protein to efficiently boost the immune response? Our MEGACD40L® Protein is what you probably need. MEGACD40L® is a chimeric protein in which two trimeric CD40 ligand molecules are artificially linked. The resulting oligomer is highly active and does not require the use of an enhancer, providing a simple and equally potent alternative to [CD40L+enhancer] combinations (see figure 3).
Do you have questions on the available tools for your immunology research? Do you need bits of advice to set up your experiment? Want to learn more about our portfolio? Do not hesitate to reach to our Technical Support Team. We will be happy to assist!
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?