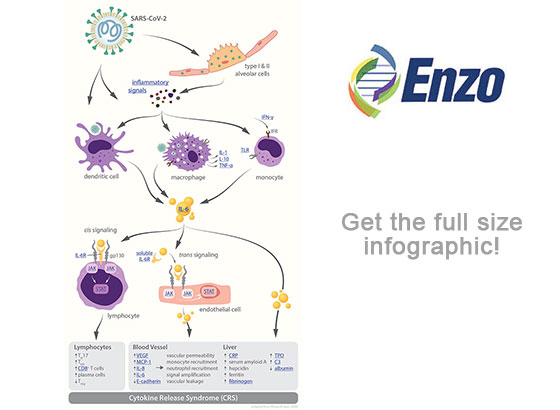
Emerging data suggest that many patients infected with COVID-19 may die due to an excessive response of their immune system, characterized by the abnormal release of circulating cytokines, termed cytokine release syndrome (CRS). CRS plays a major role in the deterioration of COVID-19 patients, from pneumonia through acute respiratory distress syndrome (ARDS), cumulating in systemic inflammation and ultimately multi-system organ failure. This phenomenon of a plethora of cytokines wreaking havoc throughout the body is often vividly referred to as “cytokine storm.”
Many cytokines take part in the “cytokine storm” in COVID-19 patients, including IL-6, IL-1, IL-2, IL-10, TNF-α and IFN-γ; however, a crucial role seems to be played by IL-6, whose increased levels in the serum have been correlated with respiratory failure, ARDS, and adverse clinical outcomes.
IL-6 has significant pro-inflammatory properties, and it functions through two main signaling pathways: cis or trans. In cis signaling, IL-6 forms a complex with the membrane-bound IL-6 receptor (mIL-6R) and gp130 which then activates downstream the Janus kinases (JAKs) and signal transducer and activator of transcription 3 (STAT3). The activation of this signal cascade leads to pleiotropic effects on the acquired immune system (B and T cells) as well as the innate immune system (neutrophils, macrophages, and natural killer cells) which can contribute to CRS. In trans signaling, high circulating IL-6 concentrations bind to the soluble form of IL-6 receptor (sIL-6R) and form a complex with a gp130 dimer on most somatic cell types. The resultant IL-6–sIL-6R–JAK-STAT3 signaling is then activated in cells that do not express mIL-6R, such as endothelial cells. This severely aggravates the “cytokine storm” through secretion of vascular endothelial growth factor (VEGF), monocyte chemoattractant protein–1 (MCP-1), IL-8, and additional IL-6, as well as reduced E-cadherin expression on endothelial cells. Secretion of VEGF and reduction of E-cadherin expression contribute to vascular permeability and leakage which participate in the pathophysiology of hypotension and pulmonary dysfunction in ARDS.
Previous studies have demonstrated the efficacy of IL-6–IL-6R antagonists for the treatment of CRS and secondary hemophagocytic lymphohistiocytosis (sHLH) which both feature serum cytokine upregulation. This suggests a crucial role for IL-6 in the pathophysiology of cytokine-driven hyperinflammatory syndromes and classifies IL-6 as a potential target for COVID-19 targeted therapy. In fact, IL-6 and IL-6R antagonists are under way for clinical trials for the management of COVID-19 patients with severe respiratory complications.
All this evidence highlights the importance of the “cytokine storm” and particularly IL-6 and its downstream signaling pathways in COVID-19 disease. An accurate detection and monitoring of all those components are crucial for a better understanding of the disease progression and for assessing the therapeutic response. Enzo offers a plethora of products that would help the scientific communities in tackling these challenges, including our IL-6 (human) High Sensitivity ELISA kit and many other cytokine ELISA assays. Additionally, we provide a list of antibodies, recombinant proteins and small molecule inhibitors to further elucidate the downstream signaling pathway activated by the “cytokine storm.”
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
