Contamination Monitoring
Ensure the quality and safety of your product(s)

Latest Articles

Home Biopharma Manufacturing Contamination Monitoring
Ensure the quality and safety of your product(s)

Biomanufacturing processes are highly complex and involve many steps, from cell culture to purification, where each step poses contamination risks. Understanding potential sources and implementing rigorous monitoring is vital to maintain product safety and quality. Contamination, from various sources can impair product integrity and efficiency. Therefore, robust monitoring protocols, including culture-based, molecular, immunological, and physical/chemical methods, are essential throughout the workflow. Immunoassays play a key role in detecting contaminants, ensuring product safety, regulatory compliance, and research integrity.
Contamination in biopharmaceuticals can occur at different stages of production and includes sources such as microorganisms, adventitious agents, and chemical impurities. Effective monitoring and control play a critical role in ensuring the safety and integrity of biopharmaceutical products while meeting rigorous regulatory standards.
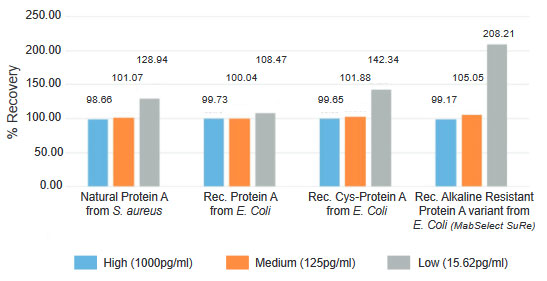
The Protein A ELISA kit is a sensitive and reproducible sandwich immunoassay to quantify Protein A residuals in monoclonal antibody preparations.
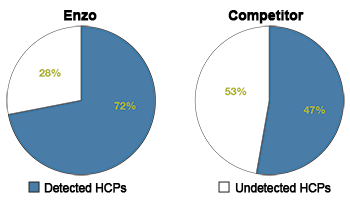
The Host Cell Protein ELISA kits and reagents are ideal for impurity analysis. Quantitatively measure host cell protein contamination in E.coli, CHO, and HEK293T derived products.

Are you in the bioprocessing field?
If so, let Enzo help you navigate the daunting quality control aspects of your operation by providing 7 simple tools to maximize your bioprocess pipeline. Whether you work in drug discovery, upstream, or downstream bioprocessing, Enzo offers a range of products to help you optimize product integrity, monitor contamination, and maximize yield.
Who should attend this webinar?
Anyone in the bioprocessing field who wants to know more about characterizing their biologic, protein aggregation, formulation optimization, storage and packaging of their biologic, and how to monitor and detect contamination.
Why should you attend this webinar?
To ensure that you are optimizing your bioprocess pipeline to facilitate rigorous and reproducible quality control in order to produce the highest quality product while consistently and reliably scaling your operation.
Presented by: Heather Brown, PhD
Application Scientist
Enzo

Do you want to visit the website?
The products in your cart will be removed.