Manufacturing
Enabling Enhanced Production, Efficient Purification, and Preservation of Product Integrity
Process Optimization for Biologics Production
In the field of biopharmaceutical production, we understand that achieving optimal results is paramount. That’s why we prioritize process optimization to unlock its full potential. Our approach revolves around three crucial aspects: enhanced production, efficient purification, and the preservation of product integrity. Optimizing bioprocess development is critical for overcoming potential challenges stemming from cell stress, cell death, protein aggregation, contamination, and other factors that might impede the reliable manufacturing of the drug. We aim to streamline and enhance your bioprocessing workflow by providing cutting-edge solutions and technologies, leading to more efficient and successful biotherapeutic development.
Enabling your Manufacturing
Key to producing cost-effective and efficient biopharmaceuticals
Safeguarding Product Integrity in Bioprocessing for Uncompromising Quality
Minimize the risk of contamination-related setbacks
Featured Applications
Key to Producing Cost-effective and Efficient Biopharmaceuticals
Maximizing yield in biopharmaceutical production is crucial for optimizing productivity and cost-effectiveness. It involves strategic process optimization, such as enhancing cell culture conditions, optimizing fermentation parameters, and improving downstream purification techniques.

Minimize the Risk of Contamination-related Setbacks
Contamination in biopharmaceuticals can occur at different stages of production and includes sources such as microorganisms, adventitious agents, and chemical impurities. Effective monitoring and control play a critical role in ensuring the safety and integrity of biopharmaceutical products while meeting rigorous regulatory standards.
Protein A ELISA Kit
The Protein A ELISA kit is a sensitive and reproducible sandwich immunoassay to quantify Protein A residuals in monoclonal antibody preparations.
- Highly sensitive measurement, detecting as little as 9.01 pg/ml (<1 ppm) of Protein A residuals in purified humanized mAb preparations
- Universally used as it recognizes 4 commonly used constructs of Protein A
- High-throughput format with results in under 3 hours for up to 37 samples in duplicate
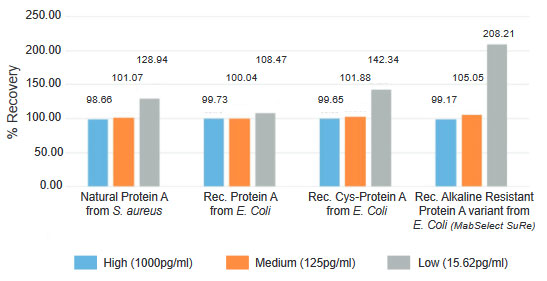
Recognition of all Commonly Used Protein A Constructs Ensures Accurate Results
Guide to Optimizing Protein Characterization

Bioprocess E-book
- Current Food and Drug Administration Guidelines
- How to Determine Protein Stability
- Methods for Detecting Protein Aggregates
- Development and Specificity of the PROTEOSTAT® Dye
- Using PROTEOSTAT® for a Rapid Detection and Characterization of Protein Aggregates by Flow Cytometry
- Detection of Bacterial Aggregates by Flow Cytometry
- Using PROTEOSTAT® Reagents to Predict Aggregation
- Propensity and Monitor Aggregation of Antibody-Drug Conjugates (ADC)

GMP Services
Your reliable partner. Delivering quality products on time.
ISO 9001 & ISO 13485
Latest Articles


 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?