AMPIPROBE® Nucleic Acid Detection

A Low-Cost Alternative
For Nucleic Acid Detection

Enzo’s mission is to provide low-cost alternative solutions to the diagnostic market. Our AMPIPROBE® platform uses novel PCR design to enable the quantification of nucleic acids for clinically relevant targets. Incorporating our IP into the AMPIPROBE® assays allows Enzo to offer a low-cost option for nucleic acid detection in a price sensitive market.
AMPIPROBE® assays are easily adaptable for laboratory use and cost-effective, without compromising on quality and performance. Compatible on open PCR platforms, AMPIPROBE® assays can be validated on existing instrumentation, eliminating the need for capital expenditures.
Enzo’s AMPIPROBE® Technology
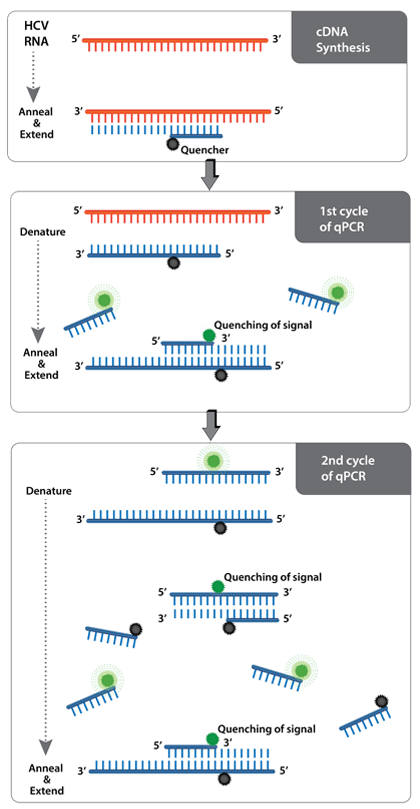
AMPIPROBE® is a proprietary technology that incorporates probe detection in primer design. It employs a combination of fluorescent reporter-labeled primers and quencher-labeled primers to amplify DNA, akin to traditional qPCR.
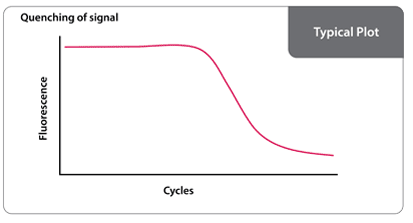
When free in solution, fluorescent primers generate a signal. However, as the primers are incorporated into amplified DNA, the quencher and the fluorophore are brought within close proximity and exhibit Forster resonance energy transfer (FRET). This causes a logarithmic decay of signal with respect to the number of amplification cycles of DNA.
Once the signal decays to a defined threshold, a value is generated with respect to the corresponding cycle. The threshold cycle is indicative of the amount of target RNA or DNA in the sample.
The AMPIPROBE® HCV assay kit is Enzo’s landmark AMPIPROBE® assay for the quantitation of HCV viral load. It has been validated and approved by the New York State Department of Health to be used as a laboratory developed test to quantitatively measure HCV viral load.
AMPIPROBE® SARS-CoV-2 Test System (RUO)
Our AMPIPROBE® SARS-CoV-2 Assay Kit (RUO) is a multiplexed assay designed for the detection of the SARS-CoV-2 virus. It contains two primer/probe sets specific to different SARS-CoV-2 genomic regions and primers/probes for internal control and negative control, human RNase P. This kit is intended to be used in combination with general RNA extraction kits, including AMPIXTRACT™ SARS-CoV-2 Extraction Kit (RUO) and generic qPCR instruments. This assay can also be used in manual settings or with open automated platforms such as the GENFLEX® Automated Instrument (please contact us for more information) or any other open platforms available on the market for the liquid handling process of RNA extraction and PCR set up.
Performance
- Sensitivity: 98.1%
- Clinical Specificity: 99.3%
- Size: 96 tests
Cost-Effective
Low-cost alternative to other methods of viral load detection
Easily Adaptable
- Adaptable to both automated open platforms (including GENFLEX®) and manual processing
AMPIPROBE® Women’s Health Pane
The AMPIPROBE® Women’s Health Panel is a highly sensitive and specific multiplex nucleic acid amplification assay developed with Enzo’s proprietary technology. It allows for the detection of 16 pathogens, including Bacterial Vaginosis (BV), Trichomoniasis (TV), Vulvovaginal Candidiasis (VVC), Ureaplasma and Mycoplasma (UMM), as well as sexually transmitted infections such as Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG). Designed to be flexible, the AMPIPROBE® Women’s Health Panel consist of 5 different assays that can be performed either singularly or in a panel, according to customer need. Additionally, all the assays are adaptable to automated platforms, such as the Enzo’s GENFLEX™ Molecular System or any open platforms available, or to manual settings.
AMPIPROBE® Women’s Health Panel
C. albicans
C. glabrata
C. krusei
C. parapsilosis
C. tropicalis
Chlamydia trachomatis
Neisseria gonorrhoeae
Trichomonas vaginalis
Atopobium vaginae
Gardnerella vaginalis
Lactobacillus spp.
Megasphaera-1
BVAB-2
Ureaplasma spp.
Mycoplasma genitalium
Mycoplasma hominis
AMPIPROBE® HCV Assay Kit
The AMPIPROBE® HCV assay kit is Enzo’s landmark AMPIPROBE® assay for the quantitation of HCV viral load. It has been validated and approved by the New York State Department of Health to be used as a laboratory developed test to quantitatively measure HCV viral load.
Key Benefits
- Sensitive quantitation of HCV viral load
- LOD Serum = 5.5 IU/mL
- LOD Plasma = 7.9 IU/mL
- Low-cost alternative to other methods of viral load detection
- Compatible with most open qPCR platforms
- Complete set of controls including:
- High, Medium and Low quantitation controls
- Internal sample extraction control
- Negative sample control
- Smaller sample input allows remaining extracted samples to be used in other tests
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?