Ubiquitin & UBL Signaling
Understanding the Ubiquitin-proteasome Pathway
Discover our Ubiquitin Solutions
Browse hundreds of high-quality research products, including proteins and antibodies, for activating enzymes (E1s), conjugating enzymes (E2s), ligases (E3s) and deconjugating enzymes (DCEs), plus an extensive range of ubiquitin-, SUMO-, NEDD8-, ISG15- and FAT10-associated proteins and derivatives. Discover ubiquitin- and Ubl-reactive antibodies, kits for the study of ubiquitin, SUMO, and NEDD8 conjugation, and our easy-to-use UBIQAPTURE® and SUMO-QAPTURE® kits for the detection and isolation of ubiquitinylated and SUMOylated proteins.
Ubiquitin
Ubiquitinylation of cellular proteins is a highly complex and tightly regulated process that targets, in a specific manner, thousands of cellular proteins. It is carried out by a modular cascade of enzymes with high specificity towards target proteins. Conjugation of ubiquitin can serve a variety of non-proteolytic functions, including activation of enzymes, modulation of membrane dynamics, or routing of the tagged proteins to their sub-cellular destination (Figure 1).
Ubiquitinylation Pathway
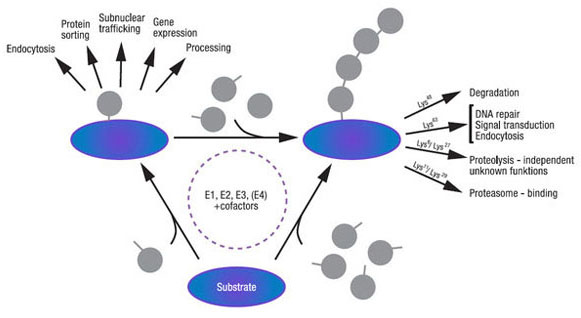
Figure 1. Differing ubiquitin modification resulting in distinct functions.
Structure of Ubiquitin
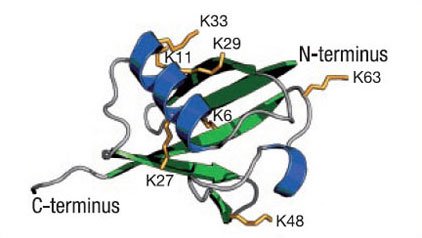
Figure 2: Lysine location within ubiquitin
Types of Ubiquitinylation
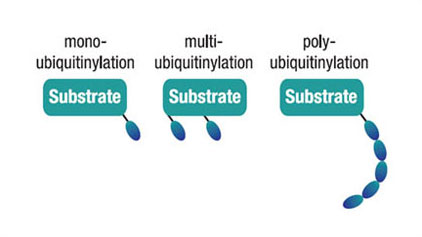
Figure 3. Ubiquitin modification
Ubiquitin-like Proteins
Ubl’s encompass a family of proteins that share structural and evolutionary relationships with ubiquitin. Ubl’s can be divided into two types based on whether they are conjugated to substrates: type I Ubl’s are conjugated and type II Ubl’s are not.
Type I Ubl’s are activated and conjugated to substrates and include the SUMO, NEDD8, ATG8, ATG12, URM1, UFM1, FAT10, and ISG15 protein families. Ubl conjugation plays diverse roles in cellular processes, including Protein Degradation, DNA Repair, Signal Transduction, Cellular Localization and Trafficking. There is a growing body of data implicating the dysregulation of Ubl-substarte modification and mutations in the Ubl-conjugation machinery in the etiology and progression of a number of human diseases.
Ubl Cascade Enzymes (E1s, E2s and E3s)
The complexity of the ubiquitin and ubiquitin-like protein cascades is considerable. In mammals, there are some ten activating enzymes (E1s) known, some twenty plus conjugating enzymes (E2s), over eight hundred ligases (E3s), and approaching one hundred deconjugating enzymes. These varied components work in a hierarchical context and, for appropriate modification with ubiquitin or a Ubl to occur, the correct combination of E1, E2, E3, substrate, and deconjugating enzyme must all work in concert. The cascades for the ubiquitin-like proteins appear not to be as complex as that of ubiquitin with a reduced number of component possibilities.
Ubiquitin Cascade
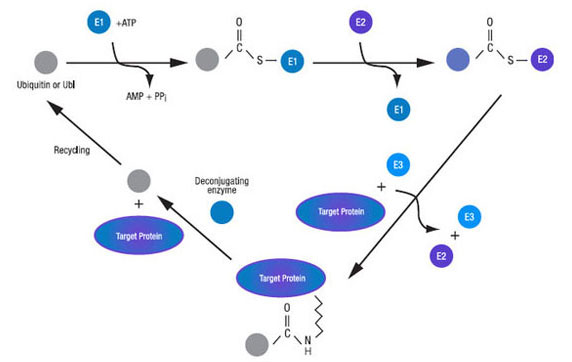
Figure 4. Activation, conjugation, ligation, deconjugation, and recycling steps.
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?