Enhanced TNF Ligands

Engineered Ligands Improves Stablity and Immune Activation
The critical role of signals transmitted via TNF ligands and receptors in mediating inflammation, cancer, and immunity has been well established in the literature. This understanding has been harnessed on the drug development frontier to mediate the pro-inflammatory properties of TNF signaling using ligand-neutralizing antibodies (eg. Remicade®) or soluble receptors (eg. Enbrel®). Similar strategies have sought to use recombinant TNF ligands to enhance, for instance, anti-viral, and anti-cancer immune activation.
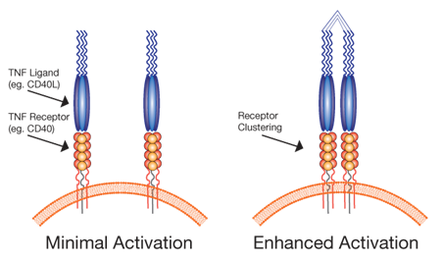
One limitation of this strategy is the inherent instability of recombinant ligands, and their inability to effectively mimic the natural clustering of TNF receptors on the cell surface required for immune activation. Oligomerization of TNF ligands using enhancers (eg. cross-linking antibodies), and expression of self-oligomerizing chimeric proteins (eg. Enzo MEGACD40® ligands) have been shown to improve stability and significantly enhance immune activation compared to that achieved with recombinant ligands alone (see figure below).
The TNF Ligand Activation
High-Quality Enhanced Ligands Deliver Consistent Results
Enzo offers a diverse collection of enhanced ligands for use in immune activation studies. Our experience in the design and large-scale production of complex, high-purity, low endotoxin protein constructs results in enhanced ligands with the purity, stability, and consistency to deliver results you can trust for the duration of your study.
Oligomerization Domains in Enzo Enhanced Ligands:
- MEGACD40L® Ligands – use the ACRP30headless domain from human or mouse ACRP30
- KILLER® Ligands – use a proprietary linker peptide that promotes trimerization
- SUPERKILLER® Ligands – use the KILLER® linker peptide mutated to increase disulfide-mediated cross-linking
Enhance Immune Activation with MEGACD40L® Protein
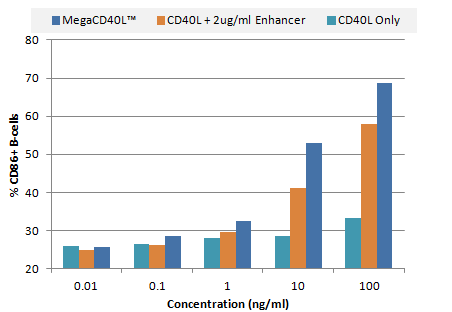
B cell lymphocyte activation by various CD40L constructs. PBMCs were treated for 48 hrs in serum free media containing serially diluted MEGACD40L® Protein (ALX-522-110), CD40L + 2ug/mL Enhancer, or CD40L. Cells were dual-stained with anti-human CD19–PE and anti-human CD86–APC and analyzed by flow cytometry. Data are presented as the percent of CD19+ B cells that co-stain as CD86+.
SUPERKILLERTRAIL™ Protein (soluble) (human), (recombinant)
Enhanced ligand that utilizes the KILLERTRAIL™ linker peptide mutated to increase disulfide-mediated cross-linking to form a more stable oligomer
- Improves stability
- Enhances immune activation compared to other recombinant ligands
Comparison of pro-apoptotic activities of human and mouse SUPERKILLERTRAIL™ Protein, Soluble (human) (rec.) on human tumor cell lines Jurkat and CEM.
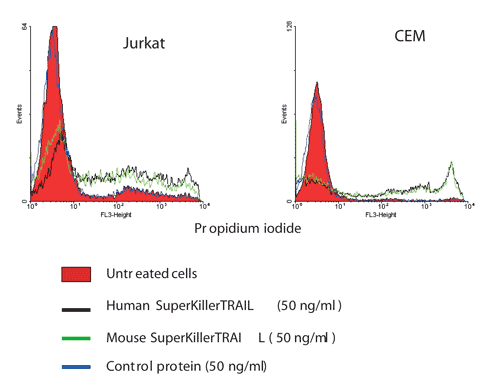
Method: Cells were treated with mouse and human SUPERKILLERTRAIL™ Protein, Soluble (human) (rec.) (CC-mutant) (50ng/ml) for 14 hours, stained with propidium iodide and analyzed by flow cytometry.
Featured References
TNF superfamily in inflammatory disease: translating basic insights. M. Croft, et al.; Trends Immunol. 33, 144 (2012).
Signaling pathways of the TNF superfamily: a double-edged sword. B. Aggarwal; Nat. Rev. Immunol. 3, 745 (2003).
A soluble hexameric form of CD40 ligand activates human dendritic cells and augments memory T cell response. I. Miconnet and G. Pantaleo; Vaccine. 26, 4006 (2008).
Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. N. Holler, et al.; Mol. Cell. Biol. 23, 1428 (2003).
Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus ventors MVA and NYVAC expressing HIV antigens. C.E. Gomez, et al.; Vaccine 27, 3165 (2009).
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?
