Prostaglandin Analysis

Enzo Life Sciences offers the most sensitive and complete colorimetric ELISA kits for quantification of prostaglandins in a wide variety of sample types.
- Ultrasensitive colorimetric ELISAs to measure as little as 8.26 pg/ml PGE2
- Widely cited in peer reviewed literature
- Available to use for cell lysates, culture supernatants, serum, saliva, urine, and many more sample types
- High throughput capabilities with chemiluminescent and fluorescent format options
- Proven manufacturing capabilities for reliable lot-to-lot results
PGE2 ELISA Kits
| Product Number | Size | Sensitivity | Time to Answer | Sample Types | |
|---|---|---|---|---|---|
| Colorimetric | ADI-900-001 | 96 wells | 13.4 pg/mL | <3 hours | Culture supernatants, serum, saliva, urine, whole blood, and more |
| ADI-901-001 | 5×96 wells | 13.4 pg/mL | <3 hours | Culture supernatants, serum, saliva, urine, whole blood, and more | |
| ADI-930-001 | 96 wells | 8.26 pg/mL | Overnight | Culture supernatants, serum, saliva, urine, whole blood, plasma | |
| ADI-931-001 | 5×96 wells | 8.26 pg/mL | Overnight | Culture supernatants, serum, saliva, urine, whole blood, plasma | |
| Fluorescent (FPIA) | ADI-920-001 | 100 tests | 684 pg/mL | 30 min | Culture supernatants |
| Chemiluminescent (CLIA) | ADI-910-001 | 96 wells | 6.03 pg/mL | 3 hours | Culture supernatants, serum, saliva, urine, whole blood |
Featured Prostaglandin Citations
- W.Y. Choi, et al., ‘Anti-inflammatory, antioxidative and matrix metalloproteinase inhibitory properties of 20(R)-ginsenoside Rh2 in cultured macrophages and keratinocytes’, (2013) J. Pharm. Pharmacol., v.65, p.310.
- T. Li, et al., ‘Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity’, (2013) Med. Oncol., v.30, p.663.
- Y. Mao, et al., ‘Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms’, (2013) Cancer Res., v.73, p.3877.
- A. Thakur, et al., ‘Microenvironment generated during EGFR targeted killing of pancreatic tumor cells by ATC inhibits myeloid-derived suppressor cells through COX2 and PGE2 dependent pathway’, (2013) J. Transl. Med., v.11, p.35.
- M.S. Pour, et al., ‘Prostaglandin E2 alteration in contraceptive consumers: as a risk factor for inflammatory diseases’, (2013) Arch. Gynecol. Obstet., v.287, p.1031.
- G.M. Park, et al.,’XH-14, a novel danshen methoxybenzo[b]furan derivative, exhibits anti-inflammatory properties in lipopolysaccharide-treated RAW 264.7 cells’, (2013) J. Inflamm., v.10, p.1
More Prostaglandin ELISA Kits
Lot-to-lot Consistency
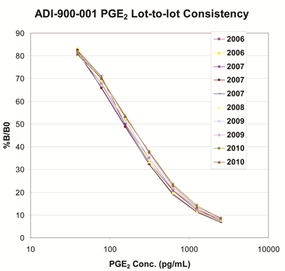
Lot-to-lot consistency graph demonstrates the robust and reproducible nature of the PGE2 ELISA kit showing standard curves from 10 lots manufactured over 5 years.
Biological Significance of Prostaglandins
The prostaglandins, together with the thromboxanes and prostacyclins, are fatty acid derivatives and a subclass of eicosanoids. Like all eicosanoids, the prostaglandins contain 20 carbon atoms and function as locally acting messenger molecules. They are produced at discrete sites acting as autocrine or paracrine factors rather than endocrine hormones. In the body, they mediate a variety of strong physiological effects, such as regulating the contraction and relaxation of smooth muscle tissue.
Prostaglandin E1 (PGE1) is synthesized from DGLA, dihomo-γ-linolenic acid and has been shown to have a number of biological actions, including vasodilation, proliferation of vascular smooth muscle cells, platelet aggregation and has been shown to have insulin-like actions. PGE1s effects are induced by receptor mediated elevation of cAMP.
Prostaglandin F2α (PGF2α) is formed in a variety of cells from PGH2, which itself is synthesized from arachidonic acid by the enzyme prostaglandin synthetase. PGF2α is often viewed as an antagonist to PGE2 due to their opposing effects on various tissues. It is a potent bronchoconstrictor and has been implicated in asthma attacks. PGF2α is also involved in reproductive functions including corpus luteum regulation, uterine contractions, and sperm motility. This has led to its use in terminating pregnancies and inducing labor at term. High levels of PGF2α have also been associated with pre-eclampsia.
Prostaglandin E2 (PGE2) is formed in a variety of cells from PGH2, which itself is synthesized from arachidonic acid by the enzyme prostaglandin synthetase. PGE2 has been shown to have a number of biological actions, including vasodilation, both anti- and proinflammatory action, modulation of sleep/wake cycles, and facilitation of the replication of human immunodeficiency virus. It elevates cAMP levels, stimulates bone resorption, and has thermoregulatory effects. Additionally, PGE2 has been shown to be a regulator of sodium excretion and renal hemodynamics.
Related Products
Prostaglandin Antibodies
Prostacyclin
Prostaglandin D2
Prostaglandin E1
Prostaglandin E2
Prostaglandin F2
Prostaglandin J2
Prostaglandin Receptor
High-purity Prostaglandins
Prostaglandin A1
Prostaglandin A2
Prostaglandin B1
Prostaglandin B2
Prostaglandin D2
Prostaglandin E1
Prostaglandin E2
Prostaglandin F2α
Prostaglandin F2α . tromethamine
Prostaglandin F1α
Prostaglandin I2 sodium
6-Ketoprostaglandin F1α
9β,11α-Prostaglandin F2
9α,11β-Prostaglandin F2
19(R)-Hydroxyprostaglandin E2
2,3-Dinor-6-keto-prostaglandin F1α-20,20,20-d3
17-Phenyl-trinor-prostaglandin E2
13,14-Dihydro-prostaglandin E1
15-Deoxy-Δ12,14-prostaglandin J2
Prostaglandin H2
 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?