Silvia Ávila1, José-Luis Castrillo1, Morgan Mathieu2
1Genetadi Biotech, Bilbao, Spain
2Enzo Life Sciences, Lausen, Switzerland
INTRODUCTION
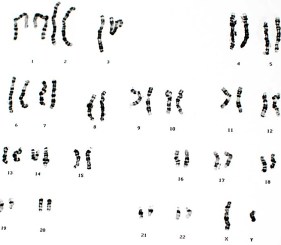
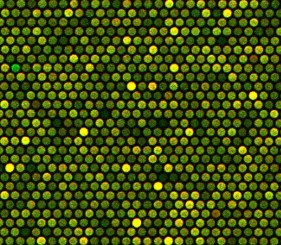
Genetadi Biotech is a company specialized in the development of new tools for genetic diagnosis in the fields of gynecology and oncology. Among the services offered by this laboratory is a technique referred to as array comparative genomic hybridization (aCGH). This method is used to identify genetic disorders such as aneuploidy, unbalanced aberrations, and amplification on a minimal amount of DNA in a variety of samples. Compared to more traditional techniques such as karyotyping and fluorescence in situ hybridization, CGH offers improved resolution in a rapid and high-throughput fashion.
The main objective of this study was to look at the ability of Enzo’s CYTAG® CGH Labeling Kit to label genomic DNA prior to hybridization on Agilent’s SurePrint G3 human 1x1M microarrays. Quality control (QC) metrics relevant to the experiment were analyzed and were deemed to be within the accepted range. Derivative log ratio scores (DLRS) were lower than 0.2, thereby reducing the need for experimental repeats, while allowing the identification of true genetic variations and validating the use of Enzo’s CYTAG® CGH Labeling Kit with Agilent’s SurePrint G3 human CGH 1x1M microarrays.
Download this Application Note
Latest Articles



 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?