Jean-Michel Lapierre1, Valérie Malan1, Rosaria Esposito2, Morgan Mathieu2
1Hôpital Necker-Enfants Malades, Paris, France
2Enzo Life Sciences, Lausen, Switzerland
INTRODUCTION
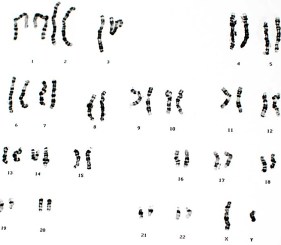
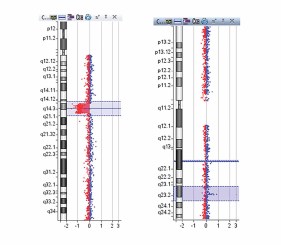
Microarray-based comparative genomic hybridization (array CGH) is a fast genome-wide screening tool for the detection of a large number of chromosomal anomalies in the form of copy number variations (CNVs).
The cytogenetic department of the Hôpital Necker-Enfants Malades in Paris (France) carries out hundreds of prenatal, postnatal, and oncological CGH tests per year on a variety of specimens, including amniotic fluid, blood, bone marrow, fresh and formalin-fixed paraffin embedded (FFPE) tissues. Since the introduction of an automated liquid handling workstation (Freedom EVO 100, Tecan), this service handles almost 2,000 samples per year, keeping its usual high standards of reproducibility and security.
The objective of this study was to verify the compatibility of Enzo’s CYTAG® SuperCGH Labeling Kit with Tecan’s automated instrument.
Download this Application Note
Latest Articles


 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?