Brad Larson1, Arturo Gonzalez-Moya2, Alexandra Wolff3, Wini Luty3
1BioTek Instruments, Inc. Winooski, VT USA
2Cisbio Bedford, MA USA
3Enzo Life Sciences Farmingdale, NY USA
INTRODUCTION
According to literature references, Extracellular-Signal Regulated Kinase (ERK) signaling has been linked to memory and regulated by environmental stimuli. In Alzheimer’s disease, progressive cognitive impairment is seen as a classic characteristic. These deficits are believed to be the result of progressive synaptic dysfunction initiated by aggregated β–amyloid peptide 1-42 (Aβ). Zhu et al. (2002) showed that Aβ induced disruption of kinases critical for memory. In late stages of Alzheimer’s disease, ERK activation is suppressed relative to early stages and controls (Webster et al. 2006). In vitro studies have also shown that under certain conditions Aβ or fragments inhibit ERK or the downstream cAMP response elementbinding protein (CREB) in neuronal cell models (Daniels et al. 2001).
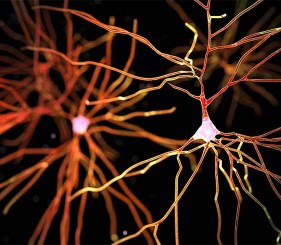
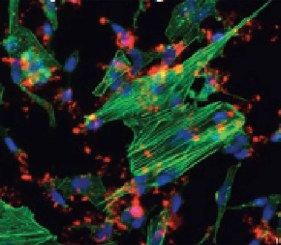
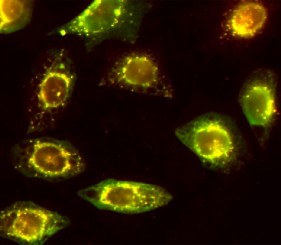
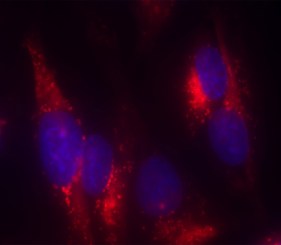
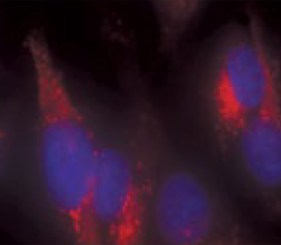
Here we evaluate the ability to detect changes in phosphorylation levels of ERK and CREB following treatment with Aβ using the SH-SY5Y neuroblastoma cell line. Aβ was oligomerized using a method previously described in the literature (FA et al. 2010). A two-step HTRF® assay process was incorporated such that cell plating and treatment are carried out in a 96-well clear-bottom imaging plate. Following lysis, aliquots were transferred to separate LV384-well assay plates to perform the phospho- and total ERK and CREB assays. A neutralizing antibody was also tested for its capacity to counteract the inhibitory effects of Aβ. Aβ binding and antibody neutralization were detected via immunocytochemistry and microscopic analysis. All microplate reading and cellular imaging steps were performed using a novel cell imaging multi-mode reader. The combination provides an efficient, robust method for testing of new molecules to combat the degenerative effects of the disease.
Download this Application Note
Latest Articles

 Lab Essentials
Lab Essentials AMPIVIEW® RNA probes
AMPIVIEW® RNA probes Enabling Your Projects
Enabling Your Projects  GMP Services
GMP Services Bulk Solutions
Bulk Solutions Research Travel Grant
Research Travel Grant Have You Published Using an Enzo Product?
Have You Published Using an Enzo Product?