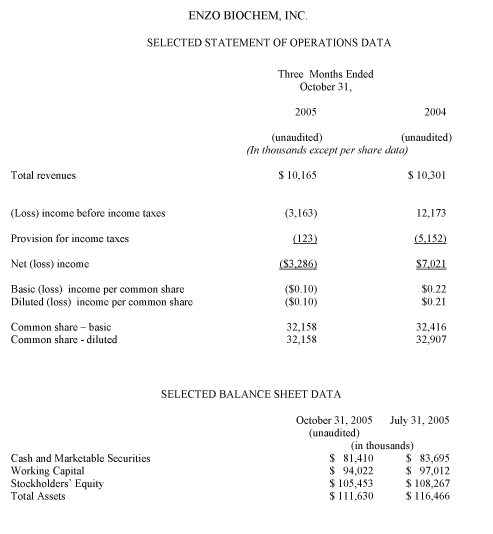
Revenues in the first quarter of fiscal 2006 were $10.2 million, compared to $10.3 million, a year ago, reflecting a $0.2 million increase in revenues at Enzo Clinical Labs and a $0.3 million decrease in revenue at Enzo Life Sciences. The net loss for the quarter totaled ($3.3) million, or ($0.10) per share, compared to net income in the comparable year-ago period of $7.0 million, or $0.21 per share. During the fiscal 2005 first quarter, the Company recorded a gain of $14.0 million as a result of a patent litigation settlement.
At the end of the fiscal 2006 first quarter, the Company's cash, cash equivalents and marketable securities amounted to $81.4 million. Working capital on October 31, 2005 stood at $94.0 million.
Enzo Clinical Labs continued to benefit from increased business in new markets, particularly in northern New Jersey. First quarter revenues at the Labs were $8.0 million, compared with $7.8 million in the year-earlier period. Gross profit declined from $4.9 million in the fiscal 2005 first quarter to $4.5 million in the fiscal 2006 first quarter. Gross profit was affected by higher operating costs related to additional personnel to support the expansion into new markets, as well as expenses to bring additional reference testing in-house and higher costs associated with esoteric tests.
At Enzo Life Sciences, fiscal 2006 first quarter revenues, which included royalty payments, were $2.1 million, compared to $2.4 million in the year earlier period. The quarter's results were impacted by not recognizing revenue from certain distributors as a result of ongoing legal issues.
Selling, general and administrative expenses for the fiscal 2006 first quarter increased to $5.5 million, from $4.1 million in the prior year’s period, reflecting higher costs in the marketing activities at Enzo Clinical Labs, increased expenses associated with compliance with Sarbanes-Oxley legislation and new governance-related procedures, and a non-cash charge of $0.3 million related to stock-based compensation. Research and development expenses declined from $2.2 million in the year-earlier period to $1.6 million in the fiscal 2006 first quarter as a result of the timing of certain clinical trials and the absence of $0.3 million relating to the amortization of patent expense that was incurred in the previous year. During the fiscal 2006 first quarter, the Company incurred legal expenses totaling $1.9 million, compared to $1.1 million a year ago. Legal expense increased as a result of higher expenses surrounding patent litigation and added patent costs associated with continued development of the Company’s intellectual property.
“While several factors impacted our financial results, we have reason to be optimistic moving forward," said Barry Weiner, President. "We are encouraged by the response we have received for certain of our genomic analysis-related products currently being evaluated by a number of opinion leaders in the field. In addition, we are currently beta testing a complete system using our proprietary dye-labeled nucleotides that is optimized for researchers who need to conveniently produce large amounts of labeled RNA for gene expression studies.
“Enzo Therapeutics continues to enroll additional subjects for an expanded Phase II clinical trial of Alequel™, the Company's immune regulation product for Crohn's disease and a Phase II study of EGS21, our immune potentiation product as a candidate therapy for non-alcoholic steatohepatitis (NASH) is ongoing. A Phase I/II study of Enzo’s StealthVector® HGTV43™ experimental gene medicine for managing HIV-1 infection is awaiting final clearance from the University of California San Francisco Institutional Review Board. We are preparing to initiate in Germany a Phase II clinical trial of our experimental drug B27PD as a treatment for autoimmune uveitis, a chronic inflammation of the eye that can often lead to blindness. This drug has been granted orphan status in Europe. We also continue to make progress on obtaining patent allowances for many of the technologies that we believe form the cornerstone of genomics today. This activity remains a high priority for the Company as we feel it will drive future additional and significant shareholder value. With the Company’s strong balance sheet and research effort, Enzo Biochem is well-positioned to further develop its intellectual property.”
About Enzo
Enzo Biochem is engaged in the research, development and manufacture of innovative health care products based on molecular biology and genetic engineering techniques, and in providing diagnostic services to the medical community. The Company's proprietary labeling and detection products for gene sequencing and genetic analysis are sold to the life sciences market throughout the world. The Company's therapeutic division is in various stages of clinical evaluation of its proprietary gene medicine for HIV-1 infection and its proprietary immune regulation medicines for hepatitis B and hepatitis C infection and for Crohn's Disease. Pre-clinical research is being conducted on several candidate compounds aimed at producing new mineral and organic bone, including technology that could provide therapy for osteoporosis and fractures, among other applications. The Company also holds a patent covering a method and materials for correcting point mutations or small insertions or deletions of genetic material that would allow for editing and correcting certain abnormalities in genes. The Company owns or licenses over 200 patents worldwide. For more information visit our website www.enzo.com.
Except for historical information, the matters discussed in this news release may be considered "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements include declarations regarding the intent, belief or current expectations of the Company and its management. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve a number of risks and uncertainties that could materially affect actual results. The Company disclaims any obligations to update any forward-looking statement as a result of developments occurring after the date of this press release.
An informational call conducted by Enzo Biochem, Inc. management will take place on Tuesday December 13, 2005 at 9:00 AM E.T. It can be accessed by dialing 1-800-921-9431. International callers can dial 1-973-935-2981. Please reference PIN number 6812867. Interested parties may also listen over the Internet at www.investorcalendar.com. To listen to the live call on the Internet, please go to the web site at least fifteen minutes early to register, download and install any necessary audio software. For those who cannot listen to the live broadcast, a replay will be available approximately two hours after the end of the live call, through midnight (ET) on December 27, 2005. The replay of the conference call can be accessed by dialing 1-877-519-4471, and, when prompted, use PIN number 6812867. International callers can dial 1-973-341-3080, using the same PIN number.